Abstract
Preliminary methodologically limited studies suggested that taste and smell known as chemosensory impairments and neuropsychiatric symptoms are associated in post-COVID-19. The objective of this study is to evaluate whether chemosensory dysfunction and neuropsychiatric impairments in a well-characterized post-COVID-19 sample. This is a cohort study assessing adult patients hospitalized due to moderate or severe forms of COVID-19 between March and August 2020. Baseline information includes several clinical and hospitalization data. Further evaluations were made using several different reliable instruments designed to assess taste and smell functions, parosmia, and neuropsychiatric disorders (using standardized psychiatric and cognitive measures). Out of 1800 eligible individuals, 701 volunteers were assessed on this study. After multivariate analysis, patients reporting parosmia had a worse perception of memory performance (p < 0.001). Moderate/severe hypogeusia was significantly associated with a worse performance on the word list memory task (p = 0.012); Concomitant moderate/severe olfactory and gustatory loss during the acute phase of COVID-19 was also significantly associated with episodic memory impairment (p = 0.006). We found a positive association between reported chemosensory (taste and olfaction) abnormalities and cognition dysfunction in post-COVID-19 patients. These findings may help us identify potential mechanisms linking these two neurobiological functions, and also support the speculation on a possible route through which SARS-CoV-2 may reach the central nervous system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SARS-CoV-2 [1], the virus responsible to cause the new coronavirus disease 2019 (COVID-19), affects several systems such as the pulmonary, cardiovascular, hematological, neurological, psychiatric, and otorhinolaryngological ones. According to recent data, around 260 million people have been infected throughout the world [2] and, of these, many individuals suffer from disease sequelae, named as long-COVID [3] or post-acute COVID-19 syndrome (PASC) [4].
Moreover, the pathophysiology of COVID-19 might be involved in the onset or aggravation of chemosensory disorders (taste and smell) [5, 6]. Besides them, parosmia which is an abnormal olfactory perception where subjects perceive differently the same smell may appear during the chemosensory loss recovery phase [6]. Although these dysfunctions are common in the early stages of infection, they are often overlooked by patients as perceived as harmlessness and common, with rates of approximately 3–20% of those who are affected by COVID-19, with a large severity range [7]. COVID-19 patients present rates of olfactory and gustatory disfunction of 41.0% and 38.2% [8], respectively, with some studies presenting prevalence as high as 83.9% [9]. Although complete recovery is common, 5% of the patients report no chemosensory recovery [10]. Interestingly, smell and taste losses were shown to be presented in 63.4% of patients underwent COVID-19 infection even after complete vaccination [11]. Parosmia, which is related to smell recovery [12], was find in 40% of COVID-19 patients assessed 6 months after the disease [13]. These sequelae may have a negative impact on the quality of life and functional capacity of survivors.
Moreover, psychiatric disorders and cognitive impairment are common acute- and post-clinical manifestations of SARS-CoV-2 infection [5, 14, 15]. Rogers et al. [16], reviewing the association between psychiatric and neuropsychiatric presentations and severe coronavirus infections, highlighted that depression, anxiety, fatigue, post-traumatic stress disorder, and rarer neuropsychiatric syndromes might develop in the longer term of the disease. Huang et al. [17] in an ambidirectional cohort study found an incidence of 23% of anxiety or depression in patients 6 months after their discharges from a hospital. Taquet et al. [5] also described a 33·62% incidence of neurological and psychiatric outcomes (e.g., dementia, mood disorder, anxiety disorder, and psychotic disorders) 6 months after SARS-CoV-2 infection. Moreover, these sequelae were more common in patients with previous SARS-CoV-2 infection than in patients who had influenza or other respiratory tract infections, stressing the impact of SARS-CoV-2 to brain homeostasis [5].
There is limited information on the association between olfactory/taste dysfunction and psychiatric symptoms in association with COVID-19. Speth et al. showed a positive correlation between severities of smell and taste loss, depression, and anxiety in a sample of COVID-19 survivors [18]. However, the study is limited to the small sample size, using only dimensional scales to depict psychiatric symptoms and no information regarding cognitive impairment. Thus, the objective of the present study is to analyze the association between olfactory and gustatory dysfunctions and neuropsychiatric morbidity, in a large cohort of moderate and severe COVID-19 recovered patients, using a large body of dimensional and structured questionnaires, as well as a systematized cognitive assessment.
Methods
Study design and population
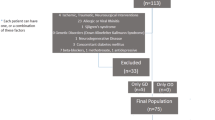
This study was carried out at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), a tertiary university hospital that has been a key element in the care of moderate to severe cases of coronavirus. All patients hospitalized at HCFMUSP for at least 24 h due to moderate or severe forms of COVID-19 between March 30th and August 30th, 2020 were regarded as eligible for this study. Moreover, we included 36 patients with highly suspected COVID-19 (based on clinical and chest-CT findings) without laboratory confirmation. These individuals had been admitted as in-patients within the first 6 weeks after the initial preparation of IC-HCFMUSP as a COVID-only facility, and the decision to include them was because the in-hospital RT-PCR testing setup was not yet fully operational at that time, thus increasing the risk of false-negative results. To a better description of the study design, please see Busatto Filho et al. [19].
In this study, we excluded those who did not complete neuropsychiatric and otorhinolaryngological batteries, presented previous diagnoses of end-stage cancer, subjects living in long-term facilities, or insufficient physical mobility to leave home after 6 months of hospital discharge, suspected reinfection at the time of follow-up and those who refused to participate in the study, thus reporting a total of 701 volunteers who signed informed consent and fulfilled the neuropsychiatric assessments between October/2020 and April/2021. This study has been approved by the Ethics Committee at HCFMUSP (CAPPesqHC), and registered at the Brazilian Registry of Clinical Trials (ReBEC) under the registration number 4.270.242 (RBR-8z7v5wc), and is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement [20].
Assessment protocol and data collection
Hospital charts and databases were used to obtain information on duration of hospital stay; requirement/duration of ICU care; requirement of orotracheal intubation, mechanical ventilation, or dialysis; and any available information about previous diagnoses, comorbidities, and relevant clinical symptoms. All assessment were made in face-to-face sequential interviews, with a team of psychiatrists, psychologists, neuropsychologists, and medical students for psychiatric and cognitive battery, and otolaryngologists, for the olfactory and taste questionnaires including visual analogue scale regarding either chemosensory, parosmia and recovery rates. To standardize procedures and maximize the reliability of the tests made, all examiners were submitted to training sections before starting the data collection. We also evaluated the global health status (visual analogue scale), physical exercise (using International Physical Activity Questionnaire [21]), and frailty—current and before COVID-19 (using the Clinical Frailty Scale [22]). Further evaluations were made using those following instruments (better descripted in Supplementary Material 1): (A) Olfactory and Taste Assessment: The evaluation of integrity of olfactory and gustatory function (according to the patients’ subjective impression) was performed with the aid of Visual Analogue Scale developed by authors, as reported in the previous studies [23, 24]. In brief, the patients were asked to indicate their perception of change in the previous ability to recognize (a) smell or (b) taste in a numeric scale ranging from 0 to 10, where higher scores represent better function [0 = unable to identify any (a) smell or (b) taste; 10 = no impairment in (a) smell or (b) taste sensitivity]. These scales were administered upon objective, multidisciplinary reassessment of patients 6–11 months after hospital discharge to depict patients’ current perception of impairment in smell or taste identification, and also retrospectively to estimate the occurrence of any such impairments during the acute phase of COVID-19. Cut-off scores were used to allocate participants into distinct categories according to magnitude of olfactory and/or gustatory impairment, i.e., severe impairment (0–4); moderate impairment (8–5); mild impairment (9); or no impairment (10) in these chemosensory functions. Subjects presenting with moderate/severe impairment were compared with those reporting mild/no impairment to verify the association of these conditions with neuropsychiatric outcomes. Subjects were also inquired about the presence parosmia in a binary question (yes/no); (B) Structured Psychiatric Interview: Clinical Interview Schedule-Revised (CIS-R), and Structured Clinical Interview for DSM-5 Disorders, Clinical Version (SCID-5-CV) for psychotic disorders; (C) Psychiatric Assessment Scales: Hospital Anxiety and Depression Scale (HAD), Ask Suicide-Screening Questions (ASQ), Post-Traumatic Stress Disorder Checklist (PCL-C), and Alcohol Use Disorder Identification Test (AUDIT); (D) Cognitive Assessment: Memory Complaint Scale (MCS), Temporal and Spatial Orientation of Mini-Mental State Examination (MMSE), Trail Making Test (TMT), digit symbol substitution test (DSST), and Neuropsychological Battery CERAD.
Statistical analysis
The sample of patients was described using frequency, mean, standard deviation, and confidence interval of demographic characteristics and clinical variables. The main variables of interest were defined as olfactory and gustatory dysfunctions, namely, parosmia; hyposmia, i.e., moderate and severe current olfactory loss (those who pointed out fewer than 8 in self-report); hypogeusia, i.e., moderate and severe current gustatory loss (those who pointed out less than 8 in self-report); and hyposmia/hypogeusia, i.e., moderate and severe current olfactory and gustatory loss (those who pointed out less than 8 in both self-reports). Univariate analyses were performed to identify covariates and factors associated with the variables of interest at a 10% significance level, since this is an exploratory study [25]. To evaluate this association in discrete factors and covariates, χ2 and Mann–Whitney tests were used, respectively. For statistical significance analysis, we adopted p value and Bonferroni adjusted p value. Multivariate analyses were performed for combinations of covariates that showed significant univariate association with the variables of interest. This association was evaluated through stepwise Logistic Regression at a significance level of 5%. The covariates and factors analyzed include sociodemographic parameters (age and gender), baseline hospitalization parameters (need of ICU, Intubation or Dialysis, length of hospitalization), social issues (financial problems following COVID-19 and Death of Close relatives), global health status (physical exercise using IPAQ questionnaire, Global health Status, and Frailty), and Psychiatric and Cognitive Measures.
Results
A total of 701 patients answered questionnaires. Table 1 describes main sample’s sociodemographics and clinical characteristics. The mean age was 55.3 years (SD: 14.6), with 52.4% of males and a mean duration of hospitalization of 17.6 days (SD: 17.6). Regarding specific care, 56.4% needed ICU care, 37.4% intubation, and 12.7% hemodialysis. Regarding the general health status, 10.1% of the subjects described their health as ‘bad or very bad’, 38.5% as ‘average’, and 51.4% as ‘good or very good’. Furthermore, 38.3% declared being sedentary, with only 3.9% of subjects perceiving themselves as ‘very active’. Interestingly, we found 12 people with olfactory hallucinations and nine individuals with gustatory hallucinations. Of those, 72.7% of subjects with olfactory and 87.5% of those with gustatory hallucinations reported that these symptoms were not present prior to COVID-19.
Hereinafter, in this paragraph, descriptive statistics of the neuropsychiatric variables will be present. First, CIS-R diagnoses prevalence of our sample are depression 7.5%; panic disorder 0.8%; agoraphobia 1.5%; social phobia 0.8%; specific phobia 2.1%; generalized anxiety disorder 15.1%; obsessive–compulsive disorder 3.1%; mixed depressive and anxiety disorder 13.5%; common mental disorder 30%. Besides CIS-R diagnosis, we found the following results on psychiatric assessment: PTSD prevalence 13.4%; last-year suicidal attempt: 2.4%; last 4 weeks suicidal ideation 10.1%; HAD anxiety mean 6.0 (SD: 5.1); HAD depression mean 4.8 (SD: 4.6); AUDIT score mean 1.56 (SD: 3.5). Regarding cognitive outputs, we found: MCS mean 5.2 (SD: 4.16); MMSE orientation score mean 8.27 (SD: 3.25); TMT-A mean 65.5 s (SD: 48.0 s); verbal fluency mean 15.57 (SD: 5.43); DSST mean 32.2 (SD: 19.3); Boston naming test mean 13.15 (SD: 2.27); word list mean 15.35 (SD: 4.7); constructional praxis mean 8.26 (SD: 2.55); word list recall mean 4.86 (SD: 2.25); and word list recognition mean 7.88 (SD: 2.77).
Moderate/severe chemosensory impairments with reported onset during the acute phase of COVID-19 were significantly associated with long-lasting moderate/severe olfactory and/or gustatory symptoms, as observed after 6–11 months of follow-up. Univariate analyses (Table 2) indicate several statistically significant associations of dependent variables with the distinct subtypes of chemosensory impairment (olfactory, gustatory, or concomitant olfactory/gustatory impairment). Parosmia was significantly associated with the magnitude of cognitive complaints (MCS) and impairment in naming ability (Boston), as well as with the occurrence of psychiatric symptoms (ASQ) and CIS-R diagnoses (‘anxiety disorder’ and ‘common mental disorder’). Moderate/severe hyposmia was associated with older age and with worse cognitive performance, as shown by the TMT-A (longer time of execution), DDST (more incorrect answers) and CERAD’s word list memory task (small number of recalled words). Moderate/severe hypogeusia was also related to a worse performance on the memory task. Finally, patients presenting with moderate or severe impairments in both chemosensory functions (i.e., concomitant olfactory and gustatory dysfunction) were older, and had more psychiatric symptoms and a worse overall cognitive performance. In this sub-sample of post-COVID survivors, we found statistically significant associations with diagnoses of ‘mixed anxiety and depressive disorder’ and ‘common mental disorder’, and with the occurrence of memory complaints according to the MCS. These patients also had lower scores in the TMT-A, DSST, VFT, and CERAD’s word list recall.
Table 3 presents a multivariate analysis between variables showing statistically significant associations with the four a priori chosen dependent variables (i.e., parosmia; moderate/severe hyposmia; moderate/severe hypogeusia; concomitant moderate/severe hyposmia and hypogeusia). Therefore, variables identified as significant in univariate analysis (Table 2) were included in the stepwise Logistic Regression analysis. Moderate/severe chemosensory losses during the acute phase of COVID-19 remained significantly (p < 0.001) associated with current moderate/severe chemosensory losses. Patients reporting parosmia had a worse perception of memory performance (as shown by higher scores in the MCS; p < 0.001). Moderate/severe hypogeusia was significantly associated with a worse performance on the memory test (CERAD’s word list recall, p = 0.012); Concomitant moderate/severe olfactory and gustatory loss during the acute phase of COVID-19 was also significantly associated with memory impairment according to CERAD’s word list memory task (p = 0.006).
Discussion
To our knowledge, this is the first study to demonstrate associations between neuropsychiatric dysfunction with chemosensory functions (smell and taste) in a large prospective cohort of post-COVID individuals. Upon multivariate analysis, certain cognitive variables (such as subjective memory complaints and performance on the word list recall) remained significantly associated with poor post-COVID-19 olfactory and gustatory functions. Although preliminary analyses identified in association with chemosensory deficits, psychiatric symptoms (or diagnoses) did not retain statistical significance after controlling for multiple covariates in logistic regression. We found several interesting and promising associations that could help clinicians and researchers better understand the link between COVID-19, chemosensory (taste and smell impairments), and brain functions as well as to extend to other connections between olfactory and gustatory functions and neuropsychiatric symptoms.
Neuropsychiatric impairments following COVID-19 are multiple, but greater attention have been given to the cognitive function and the higher risk for dementia [5, 26]. In our sample, a worse memory perception was positively associated with parosmia 6–9 months following COVID-19 infection. Interestingly, both worse current gustatory and olfactory function were associated with a reduced performance in the word list memory test. The word list memory test evaluates episodic memory [27], a cognitive function heavily impaired in Alzheimer’s Disease (AD) and strongly related to the hippocampus and connections. It is important to stress is that episodic memory is the capacity to learn, reserve, and retrieve subjective daily life information [28], being associated with several brain structures within the hippocampus and parahippocampal regions (such as perirhinal, entorhinal, and parahippocampal cortices) [29]. Even though, in our sample, cognitive dysfunction was not associated with isolated olfactory impairment, it was significantly associated with gustatory loss and gustatory plus olfactory losses. The subdivision of between taste and smell seems to be more theoretical than practical, seen that the major cause of taste impairment is olfactory dysfunction [30].
Complex interaction of several inter-related brain structures might also explain our findings regarding chemosensory loss and decrease in memory function in long-COVID. There is a possibility that anterograde pathogenic transmission of SARS-CoV-2 infected with the olfactory system may cause symptoms in the brain [31]. Although very small but apparently, human olfactory neurons with SARS-CoV-2 infection have been reported in autopsy [32] and in vitro [33], linking chemosensory dysfunction to brain impairment. Since reaching the nervous system, SARS-CoV-2 might induce a cascade of several different cellular and molecular processes producing neuropathological impairments with similar features of some neurodegenerative diseases [34]. Anatomically, the olfactory network is involved by the pathological process of AD. It is well known that olfactory dysfunction is a common feature of AD even in its initial phase [35,36,37,38,39,40,41], possible related to the presence of beta-amyloid deposits and neurofibrillary tangles from the olfactory bulb to the brain regions that receive neuronal projections directly or indirectly from the olfactory bulb, including the piriform cortex, amygdala, hippocampal and entorhinal cortex, and orbitofrontal cortex [42, 43]. The piriform cortex has a spatial and connectivity relationship with the transentorhinal cortex, the region primarily affected in most AD cases, and with the hippocampus, the structure most directly related to episodic memory [44, 45]. This complex interaction of several inter-related brain structures might explain our findings regarding chemosensory loss and decrease in memory function in long-COVID.
Noteworthy, regarding the impact of chemosensory deficits on mental health, even though we found associations between smell and taste alterations with psychiatric diagnoses (mostly anxiety and common mental disorders), the statistical significance of these associations was not sustained upon multivariate analysis. Previous studies suggested a link between hyposmia/anosmia and the development of major depressive disorder [46,47,48,49,50,51], but apparently individuals with unipolar depression tend to recover their olfactory function after symptomatic remission, contrary to individuals with bipolar depression [52]. Neuroimaging studies suggested that smaller volumes of the olfactory bulb could be associated with depression [53, 54]. In rodents, depressive states induced by olfactory bulbectomy is related to several abnormalities in neurochemical processes in the hippocampus [55], which points out to a potential causative link.
We must acknowledge the limitations of the present study. First, although participants in this cohort were evaluated after 6–11 months after the acute phase of COVID-19, the characterization of neuropsychiatric symptoms and chemosensory symptoms at baseline was retrospective and, therefore, not provided by a standardized protocol. Nonetheless, we had access to a large body of clinical data relative to the hospital treatment phase, based on which we were able to build a substantial database to ascertain the impact of these variable on mental health outcomes. Second, given the voluntary participation in the study, one must consider that some individuals with higher degrees of cognitive and/or psychiatric impairments may have been less prone to accept enrolment or to comply with the whole assessment, which could generate a selection bias. Third, we have no objective data regarding previous participants’ mental and/or cognitive health impairments. Finally, we did not use psychophysical measures to objectively determine chemosensory symptoms; rather, we used self-response questionnaires to estimate the patients’ perception of the integrity of smell and taste abilities. Although this approach may be less accurate and prone to recall bias when estimating these functions retrospectively, we understand that the substantial size of the present sample may render this approach based on self-reported questionnaires acceptable [23].
In sum, this study is the first to characterize the association between olfactory and gustatory symptoms and neuropsychiatric status in a large cohort of post-COVID-19 individuals. We found a positive association between reported chemosensory abnormalities and few neuropsychiatric symptoms, particularly those illustrating cognition dysfunction. These findings may help us identify potential mechanisms linking these two neurobiological functions, and also support the speculation on a possible route through which SARS-CoV-2 may reach the central nervous system and lead to neurocognitive impairment thereafter. Furthermore, we suggest a stronger link between taste and cognition that deserver further investigation.
References
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clin Res Ed) 368:m1091. https://doi.org/10.1136/bmj.m1091
Medicine JHU (2020) Coronavirus resource center, United States
Baig AM (2020) Chronic covid syndrome: need for an appropriate medical terminology for long-covid and covid long-haulers. J Med Virol. https://doi.org/10.1002/jmv.26624
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY (2021) Post-acute covid-19 syndrome. Nat Med 27:601–615. https://doi.org/10.1038/s41591-021-01283-z
Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ (2021) 6-month neurological and psychiatric outcomes in 236 379 survivors of covid-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 8:416–427. https://doi.org/10.1016/S2215-0366(21)00084-5
Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, Kaur R, Liuzza MT, Pepino MY, Schöpf V, Pereda-Loth V, Olsson SB, Gerkin RC, Rohlfs Domínguez P, Albayay J, Farruggia MC, Bhutani S, Fjaeldstad AW, Kumar R, Menini A, Bensafi M, Sandell M, Konstantinidis I, Di Pizio A, Genovese F, Öztürk L, Thomas-Danguin T, Frasnelli J, Boesveldt S, Saatci Ö, Saraiva LR, Lin C, Golebiowski J, Hwang LD, Ozdener MH, Guàrdia MD, Laudamiel C, Ritchie M, Havlícek J, Pierron D, Roura E, Navarro M, Nolden AA, Lim J, Whitcroft KL, Colquitt LR, Ferdenzi C, Brindha EV, Altundag A, Macchi A, Nunez-Parra A, Patel ZM, Fiorucci S, Philpott CM, Smith BC, Lundström JN, Mucignat C, Parker JK, van den Brink M, Schmuker M, Fischmeister FPS, Heinbockel T, Shields VDC, Faraji F, Santamaría E, Fredborg WEA, Morini G, Olofsson JK, Jalessi M, Karni N, D’Errico A, Alizadeh R, Pellegrino R, Meyer P, Huart C, Chen B, Soler GM, Alwashahi MK, Welge-Lüssen A, Freiherr J, de Groot JHB, Klein H, Okamoto M, Singh PB, Hsieh JW, Reed DR, Hummel T, Munger SD, Hayes JE (2020) More than smell-covid-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses 45:609–622. https://doi.org/10.1093/chemse/bjaa041
Pallanti S (2020) Importance of sars-cov-2 anosmia: from phenomenology to neurobiology. Compr Psychiatry 100:152184. https://doi.org/10.1016/j.comppsych.2020.152184
Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R (2020) Smell and taste dysfunction in patients with covid-19: a systematic review and meta-analysis. Mayo Clin Proc 95:1621–1631. https://doi.org/10.1016/j.mayocp.2020.05.030
Sbrana MF, Fornazieri MA, Bruni-Cardoso A, Avelino-Silva VI, Schechtman D, Voegels RL, Malnic B, Glezer I, de Rezende PF (2021) Olfactory dysfunction in frontline health care professionals during covid-19 pandemic in brazil. Front Physiol. https://doi.org/10.3389/fphys.2021.622987
Brandão Neto D, Fornazieri MA, Dib C, Di Francesco RC, Doty RL, Voegels RL, Pinna FR (2021) Chemosensory dysfunction in covid-19: prevalences, recovery rates, and clinical associations on a large brazilian sample. Otolaryngol Head Neck Surg 164:512–518. https://doi.org/10.1177/0194599820954825
Mungmunpuntipantip R, Wiwanitkit V (2022) Smell and taste loss in covid-19 after complete vaccination: correspondence. Laryngoscope 132(5):E18. https://doi.org/10.1002/lary.29988
Liu DT, Sabha M, Damm M, Philpott C, Oleszkiewicz A, Hähner A, Hummel T (2021) Parosmia is associated with relevant olfactory recovery after olfactory training. Laryngoscope 131:618–623. https://doi.org/10.1002/lary.29277
Di Stadio A, D’Ascanio L, La Mantia I, Ralli M, Brenner MJ (2022) Parosmia after covid-19: olfactory training, neuroinflammation and distortions of smell. Eur Rev Med Pharmacol Sci 26:1–3. https://doi.org/10.26355/eurrev_202201_27739
Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, Coles JP, Manji H, Al-Shahi Salman R, Menon DK, Nicholson TR, Benjamin LA, Carson A, Smith C, Turner MR, Solomon T, Kneen R, Pett SL, Galea I, Thomas RH, Michael BD, Allen C, Archibald N, Arkell J, Arthur-Farraj P, Baker M, Ball H, Bradley-Barker V, Brown Z, Bruno S, Carey L, Carswell C, Chakrabarti A, Choulerton J, Daher M, Davies R, Di Marco BR, Dima S, Dunley R, Dutta D, Ellis R, Everitt A, Fady J, Fearon P, Fisniku L, Gbinigie I, Gemski A, Gillies E, Gkrania-Klotsas E, Grigg J, Hamdalla H, Hubbett J, Hunter N, Huys A-C, Ihmoda I, Ispoglou S, Jha A, Joussi R, Kalladka D, Khalifeh H, Kooij S, Kumar G, Kyaw S, Li L, Littleton E, Macleod M, Macleod MJ, Madigan B, Mahadasa V, Manoharan M, Marigold R, Marks I, Matthews P, McCormick M, McInnes C, Metastasio A, Milburn-McNulty P, Mitchell C, Mitchell D, Morgans C, Morris H, Morrow J, Mubarak Mohamed A, Mulvenna P, Murphy L, Namushi R, Newman E, Phillips W, Pinto A, Price DA, Proschel H, Quinn T, Ramsey D, Roffe C, Ross Russell A, Samarasekera N, Sawcer S, Sayed W, Sekaran L, Serra-Mestres J, Snowdon V, Strike G, Sun J, Tang C, Vrana M, Wade R, Wharton C, Wiblin L, Boubriak I, Herman K, Plant G (2020) Neurological and neuropsychiatric complications of covid-19 in 153 patients: a uk-wide surveillance study. Lancet Psychiatry 7:875–882. https://doi.org/10.1016/S2215-0366(20)30287-X
Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T (2020) Neurological associations of covid-19. Lancet Neurol 19:767–783. https://doi.org/10.1016/S1474-4422(20)30221-0
Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS (2020) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the covid-19 pandemic. Lancet Psychiatry. https://doi.org/10.1016/s2215-0366(20)30203-0
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B (2021) 6-month consequences of covid-19 in patients discharged from hospital: a cohort study. Lancet 397:220–232. https://doi.org/10.1016/S0140-6736(20)32656-8
Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR (2020) Mood, anxiety and olfactory dysfunction in covid-19: evidence of central nervous system involvement? Laryngoscope 130:2520–2525. https://doi.org/10.1002/lary.28964
Busatto Filho G, Araujo AL, Duarte AJS, Levin AS, Guedes BF, Kallas EG, Pinna FR, Souza HP, Silva KR, Sawamura MVY, Seelaender M, Imamura M, Garcia ML, Forlenza OV, Nitrini R, Damiano RF, Rocha VG, Batistella LR, de Carvalho CRR (2021) Post-acute sequelae of sars-cov-2 infection (pasc): protocol for a multidisciplinary prospective observational evaluation of a cohort of patients surviving hospitalization in São paulo, Brazil. BMJ Open 11:e051706
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395. https://doi.org/10.1249/01.Mss.0000078924.61453.Fb
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A (2005) A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 173:489–495. https://doi.org/10.1503/cmaj.050051
McCormack HM, Horne DJ, Sheather S (1988) Clinical applications of visual analogue scales: a critical review. Psychol Med 18:1007–1019. https://doi.org/10.1017/s0033291700009934
Sayin İ, Yaşar KK, Yazici ZM (2020) Taste and smell impairment in covid-19: an aao-hns anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg 163:473–479. https://doi.org/10.1177/0194599820931820
Dahiru T (2008) P - value, a true test of statistical significance? A cautionary note. Ann Ib Postgrad Med 6:21–26. https://doi.org/10.4314/aipm.v6i1.64038
de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S (2021) The chronic neuropsychiatric sequelae of covid-19: the need for a prospective study of viral impact on brain functioning. Alzheimers Dement 17:1056–1065. https://doi.org/10.1002/alz.12255
Gavett BE, Horwitz JE (2012) Immediate list recall as a measure of short-term episodic memory: insights from the serial position effect and item response theory. Arch Clin Neuropsychol 27:125–135. https://doi.org/10.1093/arclin/acr104
Dickerson BC, Eichenbaum H (2010) The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 35:86–104. https://doi.org/10.1038/npp.2009.126
Camina E, Güell F (2017) The neuroanatomical, neurophysiological and psychological basis of memory: current models and their origins. Front Pharmacol. https://doi.org/10.3389/fphar.2017.00438
Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB Jr (1991) Smell and taste disorders, a study of 750 patients from the university of pennsylvania smell and taste center. Arch Otolaryngol Head Neck Surg 117:519–528. https://doi.org/10.1001/archotol.1991.01870170065015
Boldrini M, Canoll PD, Klein RS (2021) How covid-19 affects the brain. JAMA Psychiat 78:682–683. https://doi.org/10.1001/jamapsychiatry.2021.0500
Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel H-H, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL (2021) Olfactory transmucosal sars-cov-2 invasion as a port of central nervous system entry in individuals with covid-19. Nat Neurosci 24:168–175. https://doi.org/10.1038/s41593-020-00758-5
Lyoo KS, Kim HM, Lee B, Che YH, Kim SJ, Song D, Hwang W, Lee S, Park JH, Na W, Yun SP, Kim YJ (2022) Direct neuronal infection of sars-cov-2 reveals cellular and molecular pathology of chemosensory impairment of covid-19 patients. Emerg Microbes Infect 11:406–411. https://doi.org/10.1080/22221751.2021.2024095
Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP, Fehlmann T, Stein JA, Schaum N, Lee DP, Calcuttawala K, Vest RT, Berdnik D, Lu N, Hahn O, Gate D, McNerney MW, Channappa D, Cobos I, Ludwig N, Schulz-Schaeffer WJ, Keller A, Wyss-Coray T (2021) Dysregulation of brain and choroid plexus cell types in severe covid-19. Nature 595:565–571. https://doi.org/10.1038/s41586-021-03710-0
Gjerde KV, Müller B, Skeie GO, Assmus J, Alves G, Tysnes OB (2018) Hyposmia in a simple smell test is associated with accelerated cognitive decline in early parkinson’s disease. Acta Neurol Scand 138:508–514. https://doi.org/10.1111/ane.13003
Liang X, Ding D, Zhao Q, Wu W, Xiao Z, Luo J, Hong Z (2020) Inability to smell peppermint is related to cognitive decline: a prospective community-based study. Neuroepidemiology 54:258–264. https://doi.org/10.1159/000505485
Velayudhan L, Wilson-Morkeh F, Penney E, Jesu AJM, Baillon S, Brugha T (2018) Smell identification function in early-onset alzheimer’s disease and mild cognitive impairment. Int Psychogeriatr. https://doi.org/10.1017/s1041610218001503
Suzuki Y, Yamamoto S, Umegaki H, Onishi J, Mogi N, Fujishiro H, Iguchi A (2004) Smell identification test as an indicator for cognitive impairment in alzheimer’s disease. Int J Geriatr Psychiatry 19:727–733. https://doi.org/10.1002/gps.1161
Yoshii F, Onaka H, Kohara S, Ryo M, Takahashi W (2019) Association of smell identification deficit with alzheimer’s disease assessment scale-cognitive subscale, japanese version scores and brain atrophy in patients with dementia. Eur Neurol 81:145–151. https://doi.org/10.1159/000501311
Zendehbad AS, Noroozian M, Shakiba A, Kargar A, Davoudkhani M (2020) Validation of iranian smell identification test for screening of mild cognitive impairment and alzheimer’s disease. Appl Neuropsychol Adult. https://doi.org/10.1080/23279095.2019.1710508
Scalco MZ, Streiner DL, Rewilak D, Castel S, Van Reekum R (2009) Smell test predicts performance on delayed recall memory test in elderly with depression. Int J Geriatr Psychiatry 24:376–381. https://doi.org/10.1002/gps.2132
Devanand DP (2016) Olfactory identification deficits, cognitive decline, and dementia in older adults. Am J Geriatr Psychiatry 24:1151–1157. https://doi.org/10.1016/j.jagp.2016.08.010
Son G, Jahanshahi A, Yoo SJ, Boonstra JT, Hopkins DA, Steinbusch HWM, Moon C (2021) Olfactory neuropathology in alzheimer’s disease: a sign of ongoing neurodegeneration. BMB Rep 54:295–304. https://doi.org/10.5483/BMBRep.2021.54.6.055
Saiz-Sanchez D, De la Rosa-Prieto C, Ubeda-Banon I, Martinez-Marcos A (2015) Interneurons, tau and amyloid-β in the piriform cortex in alzheimer’s disease. Brain Struct Funct 220:2011–2025. https://doi.org/10.1007/s00429-014-0771-3
Li W, Howard JD, Gottfried JA (2010) Disruption of odour quality coding in piriform cortex mediates olfactory deficits in alzheimer’s disease. Brain 133:2714–2726. https://doi.org/10.1093/brain/awq209
Swiecicki L, Zatorski P, Bzinkowska D, Sienkiewicz-Jarosz H, Szyndler J, Scinska A (2009) Gustatory and olfactory function in patients with unipolar and bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 33:827–834. https://doi.org/10.1016/j.pnpbp.2009.03.030
Hur K, Choi JS, Zheng M, Shen J, Wrobel B (2018) Association of alterations in smell and taste with depression in older adults. Laryngoscope Investig Otolaryngol 3:94–99. https://doi.org/10.1002/lio2.142
Qazi JJ, Wilson JH, Payne SC, Mattos JL (2020) Association between smell, taste, and depression in nationally representative sample of older adults in the united states. Am J Rhinol Allergy 34:369–374. https://doi.org/10.1177/1945892419897217
Sanna F, Loy F, Piras R, Moat A, Masala C (2021) Age-related cognitive decline and the olfactory identification deficit are associated to increased level of depression. Front Neurosci 15:599593. https://doi.org/10.3389/fnins.2021.599593
Eliyan Y, Wroblewski KE, McClintock MK, Pinto JM (2021) Olfactory dysfunction predicts the development of depression in older us adults. Chem Senses. https://doi.org/10.1093/chemse/bjaa075
Taalman H, Wallace C, Milev R (2017) Olfactory functioning and depression: a systematic review. Front Psychiatry 8:190. https://doi.org/10.3389/fpsyt.2017.00190
Kazour F, Richa S, Abi Char C, Surget A, Elhage W, Atanasova B (2020) Olfactory markers for depression: differences between bipolar and unipolar patients. PLoS ONE 15:e0237565. https://doi.org/10.1371/journal.pone.0237565
Rottstädt F, Han P, Weidner K, Schellong J, Wolff-Stephan S, Strauß T, Kitzler H, Hummel T, Croy I (2018) Reduced olfactory bulb volume in depression-a structural moderator analysis. Hum Brain Mapp 39:2573–2582. https://doi.org/10.1002/hbm.24024
Rottstaedt F, Weidner K, Strauß T, Schellong J, Kitzler H, Wolff-Stephan S, Hummel T, Croy I (2018) Size matters—the olfactory bulb as a marker for depression. J Affect Disord 229:193–198. https://doi.org/10.1016/j.jad.2017.12.047
Morales-Medina JC, Iannitti T, Freeman A, Caldwell HK (2017) The olfactory bulbectomized rat as a model of depression: the hippocampal pathway. Behav Brain Res 317:562–575. https://doi.org/10.1016/j.bbr.2016.09.029
Acknowledgements
We are grateful for the infrastructure support from the HCFMUSP COVID-19 taskforce (Antonio José Pereira, Rosemeire K. Hangai, Danielle P. Moraes, Renato Madrid Baldassare, Elizabeth de Faria, Gisele Pereira, Lucila Pedroso, Marcelo C. A. Ramos, Taciano Varro, and Vilson Cobello Junior) both during the baseline stage of in-hospital data collection and during the setting-up of the follow-up assessments. We are grateful for the support in organizing the logistics for the follow-up assessments of COVID-19 subjects at HCFMUSP from: Patricia Manga Favaretto, Maria Cristina Coelho de Nadai, Vivian R. B. Saboya, and other members of the Diretoria Executiva dos Laboratórios de Investigação Médica; and Michelle Louvaes Garcia and other members of the clinical research center at the Instituto do Coração (InCOr). We are also grateful to Katia Regina da Silva for creating and managing the RedCap database used for the study and Dr. Pedro Bacchi for helping analysing CIS-R data. We thank the teams led by Juliana Carvalho Ferreira, Carlos R. Ribeiro de Carvalho, Heraldo Possolo de Souza, Wilson Jacob Filho, Thiago Avelino-Silva, and José Eduardo Pompeu for the input of information into the electronic clinical database for the baseline in hospital stay of COVID-19 subjects.
HCFMUSP COVID-19 Study Group: Edivaldo M. Utiyama, Aluisio C. Segurado, Beatriz Perondi, Anna Miethke-Morais, Amanda C. Montal, Leila Harima, Solange R. G. Fusco, Marjorie F. Silva, Marcelo C. Rocha, Izabel Marcilio, Izabel Cristina Rios, Fabiane Yumi Ogihara Kawano, Maria Amélia de Jesus, Ésper G. Kallas, Carolina Carmo, Clarice Tanaka, Heraldo Possolo de Souza, Julio F. M. Marchini, Carlos R. Carvalho, Juliana C. Ferreira, Anna Sara Levin, Maura Salaroli Oliveira, Thaís Guimarães, Carolina dos Santos Lázari, Alberto José da Silva Duarte, Ester Sabino, Marcello M. C. Magri, Tarcisio E. P. Barros-Filho, Maria Cristina Peres Braido Francisco, and Silvia Figueiredo Costa.
Funding
This work was partially supported by donations from the general public under the HC-COMVIDA crowdfunding scheme (https://viralcure.org/c/hc) and the Fundação Faculdade de Medicina (ALA).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Members of the HCFMUSP COVID-19 study group are listed in the Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Damiano, R.F., Neto, D.B., Oliveira, J.V.R. et al. Association between chemosensory impairment with neuropsychiatric morbidity in post-acute COVID-19 syndrome: results from a multidisciplinary cohort study. Eur Arch Psychiatry Clin Neurosci 273, 325–333 (2023). https://doi.org/10.1007/s00406-022-01427-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01427-3