Abstract
Background
Patients with acute respiratory failure caused by cardiogenic pulmonary edema (CPE) may require mechanical ventilation that can cause further lung damage. Our aim was to determine the impact of ventilatory settings on CPE mortality.
Methods
Patients from the LUNG SAFE cohort, a multicenter prospective cohort study of patients undergoing mechanical ventilation, were studied. Relationships between ventilatory parameters and outcomes (ICU discharge/hospital mortality) were assessed using latent mixture analysis and a marginal structural model.
Results
From 4499 patients, 391 meeting CPE criteria (median age 70 [interquartile range 59–78], 40% female) were included. ICU and hospital mortality were 34% and 40%, respectively. ICU survivors were younger (67 [57–77] vs 74 [64–80] years, p < 0.001) and had lower driving (12 [8–16] vs 15 [11–17] cmH2O, p < 0.001), plateau (20 [15–23] vs 22 [19–26] cmH2O, p < 0.001) and peak (21 [17–27] vs 26 [20–32] cmH2O, p < 0.001) pressures. Latent mixture analysis of patients receiving invasive mechanical ventilation on ICU day 1 revealed a subgroup ventilated with high pressures with lower probability of being discharged alive from the ICU (hazard ratio [HR] 0.79 [95% confidence interval 0.60–1.05], p = 0.103) and increased hospital mortality (HR 1.65 [1.16–2.36], p = 0.005). In a marginal structural model, driving pressures in the first week (HR 1.12 [1.06–1.18], p < 0.001) and tidal volume after day 7 (HR 0.69 [0.52–0.93], p = 0.015) were related to survival.
Conclusions
Higher airway pressures in invasively ventilated patients with CPE are related to mortality. These patients may be exposed to an increased risk of ventilator-induced lung injury.
Trial registration Clinicaltrials.gov NCT02010073
Similar content being viewed by others
Background
Lung edema causes respiratory failure due to impairment in gas exchange and lung mechanics. In life-threatening cases, mechanical ventilation aims to maintain gas exchange until edema is resolved. However, high airway pressures may promote ventilator-induced lung injury (VILI) [1]. In patients with the acute respiratory distress syndrome (ARDS), ventilatory strategies aimed to attenuate VILI (by decreasing tidal volumes and driving pressures) have improved survival [2].
In patients with cardiogenic pulmonary edema (CPE), airspaces are flooded due to capillary congestion. Although CPE lacks an inflammatory component, its heterogeneous distribution, impaired gas exchange and respiratory mechanics and high mortality rates are shared features with ARDS [3, 4]. Mortality rates in cardiogenic pulmonary edema and cardiogenic shock remain high, with only minor improvements in the last years [5, 6]. Patients with CPE that need mechanical ventilation constitute a subgroup with a significant mortality [7,8,9], and the incidence of respiratory failure within patients admitted to cardiac intensive care units may be increasing [10, 11].
In spite of the impact of mechanical ventilation on mortality rates in patients with CPE, there is no clear evidence on the optimal ventilatory settings. Although experience with mechanical ventilation is related to better outcomes in cardiac ICUs [12], patients with CPE have traditionally been excluded from trials on mechanical ventilation and the risk of VILI has not been systematically addressed [13]. Two previous retrospective reports have associated high tidal volumes or driving pressures with mortality in patients with CPE [14, 15].
We hypothesized that patients with CPE are susceptible to VILI and their outcomes sensitive to ventilatory strategies. To test this hypothesis, patients with isolated CPE included in the LUNG SAFE study [16] were selected to study the relationships between mechanical ventilation and clinical outcomes in this population.
Methods
Study design
LUNG SAFE (clinicaltrials.gov NCT02010073) was a multicenter (459 ICUs from 50 countries), prospective cohort study that enrolled 4499 patients with hypoxemic respiratory failure [16]. Patients aged < 16 years or not willing to participate were excluded. Only patients with respiratory failure from isolated cardiac origin were included in this sub-study. All participating ICUs obtained ethics committee approval and obtained either patient consent or ethics committee waiver of consent (due to the observational nature of the study).
Data collection
Clinical data, including cause of respiratory failure, concomitant diseases and ICU and hospital outcomes were collected. Day 1 was defined as the first day meeting acute hypoxemic respiratory failure (defined as a PaO2/FiO2 ratio below 300, appearance of parenchymal abnormalities in a chest X-ray and need for ventilatory support, either invasive or non-invasive, with an airway pressure equal or above 5 cmH2O). Daily data, including ventilatory settings, gas exchange, Sequential Organ Failure Assessment (SOFA) score and concomitant respiratory therapies, were collected on days 1, 2, 3, 5, 7, 10, 14, 21, and 28 at the same hour (usually 10 AM) by the research team. Plateau pressures in patients under pressure-controlled or -assisted modes were considered equal to peak pressures.
Patient selection
Presence of heart failure at Day 1 was identified according to the responsible researcher. Methods used to confirm/discard cardiac dysfunction were collected.
Follow-up and outcomes
Patients were followed up to hospital discharge. Primary and secondary outcomes were ICU discharge alive and spontaneously breathing, and hospital mortality, respectively. Prolonged mechanical ventilation was defined as need for mechanical ventilation for more than 10 days (the 75th percentile of length of ventilation).
Statistical analysis
Data are expressed as median (interquartile range) or count (percentage). Univariable comparisons were analyzed using Wilcoxon or Chi-square tests. Differences over time between groups were assessed using a repeated measurements analysis of the variance. Correlations between ventilatory parameters and the hemodynamic component of SOFA score were assessed using Spearman’s coefficients.
Patients were classified using a latent mixture analysis in two mutually exclusive and exhaustive classes using peak, plateau and driving pressures, positive end-expiratory pressure (PEEP), tidal volume (adjusted by predicted body weight), respiratory system compliance, PaO2/FiO2 and respiratory rate on day 1. Differences in ICU survival and hospital mortality between these two classes were analyzed using a competing events model, with ICU discharge (alive and spontaneously breathing) and death as terminal events.
To identify factors related to ICU survival, inverse probability of treatment weights were calculated for driving pressure, so that each observation is weighted by the inverse of the probability of the exposure, given the observed value of other confounders [tidal volume, PaO2/FiO2 ratio, PaCO2, PEEP and history of chronic obstructive pulmonary disease (COPD)]. These weights were introduced in a marginal structural model including all available data points for a given patient, and a weighted Cox regression used to calculate the hazard ratio (HR) and its 95% confidence interval for each variable. Variables included in this Cox model were age, sex, chronic renal failure, chronic heart failure, COPD, PaO2/FiO2, PaCO2, class assigned by the latent mixture model, tidal volume (adjusted by predicted body weight), driving pressure and PEEP. By adding a time stratum, different HRs were computed for ventilatory parameters (driving pressure, tidal volume and PEEP) before and after day 7. All analyses were performed with R 4.1.0, using the packages survival [17], ipw [18], depmixS4 [19], ggplot2 [20] and ggfortify [21].
Results
From the 4499 patients with acute hypoxemic respiratory failure included in LUNG SAFE, 530 were classified as having a component of heart failure contributing to their respiratory failure. One patient was excluded due to absence of outcome data. In 138 patients, another cause of respiratory failure was registered, leaving 391 patients (age 70 [59–78], 40% female) with isolated CPE (Fig. 1A). The diagnosis of cardiac failure was supported by echocardiography (314 cases), pulmonary artery catheter (56 cases) and other techniques (49 cases). In 46 cases, diagnosis was based on clinical data only.
Median ICU and hospital stay were 5 (2–11) and 14 (5–27) days, respectively. ICU mortality was 34%, and increased up to 40% at hospital discharge (Fig. 1B and C, respectively). ICU survivors were younger and received non-invasive ventilation more frequently at admission. Non-survivors showed significantly higher airway pressures on day 1 (Table 1).
Non-invasive ventilation
Sixty-seven patients received non-invasive ventilation as first-line ventilatory therapy. There were no differences in age, sex or previous diseases between these patients and those who received invasive ventilation (Additional file 1: Table S1). Patients treated with non-invasive ventilation had lower SOFA scores, less hemodynamic impairment and lower driving pressure with the same levels of PEEP (Additional file 1: Table S1). Only 9 out of these 67 patients (13%) required invasive ventilation. ICU mortality was lower in patients who received non-invasive ventilation (15% vs 39%, p < 0.001, Additional file 1: Table S1).
Clustering by ventilatory settings
A latent mixture analysis identified two classes in invasively ventilated patients (n = 314), based on respiratory parameters at study inclusion (“high pressure” [n = 188] and “low pressure” [n = 126], Fig. 2A). Class assignment probability was below 0.7 in only 39 out of 314 patients. Fitting the model with three classes increased this number of patients up to 156. Differences between groups are shown in Table 2. Patients in the high-pressure class showed a lower probability of being discharged alive from the ICU (HR 0.79 [0.60 – 1.05], Fig. 2B), and increased hospital mortality (HR 1.65 [1.16—2.36], Fig. 2C).
A Profile plot of two patient classes identified according to several ventilatory variables at first day of invasive mechanical ventilation using a latent mixture analysis. Values show means in each variable used for classification for each class (after normalization, Z-scores). The largest differences are observed in airway pressures, whereas there are no differences in respiratory rate. B Discharge from the intensive care unit (ICU) alive and spontaneously breathing for each patient class. C Hospital mortality for each patient class. D Hazard ratios for mortality obtained from a marginal structural model addressing the time-dependent changes in each variable
The differences in ventilatory parameters over time between classes were assessed. Whereas differences in driving pressure (Fig. 3A) persisted over the first 10 days, differences in PEEP (Fig. 3B) were restricted to days 1–3. There were no differences in tidal volumes (Fig. 3C). Regarding gas exchange, there were significant differences in PaO2/FiO2 (Fig. 3D), but not in PaCO2 (Fig. 3E) or arterial pH (Fig. 3F).
Time course of ventilatory settings and gas exchange over the first 10 days, according to the previously identified latent classes. A Driving pressures. B Positive end-expiratory pressure (PEEP). C Tidal volumes, expressed as milliliters per predicted body weight (PBW). D PaO2/FiO2 ratio. E PaCO2. F Arterial pH. Values were compared between classes using a repeated measurements analysis of the variance (ANOVA)
Prolonged ventilation
Invasive mechanical ventilation was needed in 327 patients, with a median length of 4 (2 – 10) days. There were 66 patients with prolonged ventilation (more than the 75th percentile of length of ventilation). When compared to those patients with a shorter duration of mechanical ventilation (Additional file 1: Table S2), there were no significant differences in any variable collected at day 1 other than tidal volume, which was lower in patients with prolonged ventilation. The rates of mechanical circulatory support or renal replacement therapy were also similar between groups. However, development of acute respiratory distress syndrome during the ICU stay was more common in patients with prolonged ventilation.
Relationships between ventilatory settings and hemodynamics
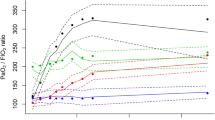
Distribution of ventilatory settings along the different levels of hemodynamic impairment (measured using the hemodynamic item of the SOFA score) was explored. Peak pressure (Fig. 4A), plateau pressure (Fig. 4B), driving pressure (Fig. 4C) and PEEP (Fig. 4D) were positively correlated with the hemodynamic SOFA score, showing weak, but significant correlations. However, no correlation was observed for tidal volumes (Fig. 4E).
Distribution of values of ventilatory settings (A Peak inspiratory pressure; B Plateau pressure; C Driving pressure; D Positive end-expiratory pressure [PEEP]; E Tidal volume) according to the hemodynamic item of the SOFA score (0: mean arterial pressure ≥ 70 mmHg; 1: mean arterial pressure < 70 mmHg, 2: dopamine ≤ 5 μg/kg/min or dobutamine; 3: dopamine 5–15 μg/kg/min or norepinephrine ≤ 0.1 μg/kg/min or epinephrin ≤ 0.1 μg/kg/min; 4: dopamine > 15 μg/kg/min or norepinephrine > 0.1 μg/kg/min or epinephrin > 0.1 μg/kg/min). Spearman’s coefficients (ρ) were calculated to assess the correlation between these parameters and the score
Role of driving pressure on survival
We fitted a marginal structural model in 248 patients with invasive ventilation and plateau pressure data. High driving pressures during the first ICU week were related to a significant increase in mortality, whereas high tidal volumes after day 7 showed the opposite (Fig. 2D and Additional file 1: Table S3). Several sensitivity analyses were performed: restricting the analysis to those patients on controlled invasive ventilation on day 1 (Additional file 1: Table S4), after exclusion of imputed plateau pressures (Additional file 1: Table S5), after excluding patients with only a clinical diagnosis of CPE (Additional file 1: Table S6), using dynamic driving pressures (measured as peak pressure minus PEEP, Additional file 1: Table S7), or inclusion of patients on non-invasive ventilation on day 1 (Additional file 1: Table S8), did not substantially modify these findings (Additional file 1: Fig. S1).
Discussion
In this sub-study of the LUNG SAFE cohort, patients with isolated cardiogenic respiratory failure showed 40% of hospital mortality, similar to that observed for ARDS patients and recent series of patients with cardiogenic shock requiring mechanical ventilation [8, 22, 23]. Our results show that high driving pressures during the first week of ventilation were associated to a significantly increased mortality, supporting the impact of mechanical ventilation on the outcomes of patients with CPE.
Alveolar edema results in reduced functional residual capacity and compliance, and may promote lung injury when high tidal volumes are applied. Alveolar flooding caused by hydrostatic, non-inflammatory mechanisms, may replicate a “baby lung” effect in patients with CPE [3]. This reduction in the airspaces available for ventilation increases the susceptibility to VILI by diverting the bulk of tidal volume towards aerated areas, causing local overdistension and increased pressures. The increased cell stretch may trigger lung inflammation, causing or perpetuating lung injury and systemic inflammation. Although the importance of VILI and the optimal ventilatory settings in patients without pre-existing inflammation remains unclear.[24], there is increasing evidence that lung inflammation may play a role in the pathogenesis of CPE and cardiogenic shock [25]. Interestingly, new onset of ARDS was the only variable related to prolonged ventilation in this cohort, highlighting the importance of a second hit on the outcome. However, the specific contribution of VILI to perpetuate cardiovascular failure, which is the most common cause of death in our cohort, is unknown.
Non-invasive ventilation can help to ensure gas exchange while avoiding intubation, and its use has yielded better outcomes in CPE [26, 27]. Our study corroborates these lower mortality rates in patients receiving non-invasive ventilation. It is unclear if these patients are exposed to an increased risk of ventilator-induced lung injury, although it has been proposed that large spontaneous inspiratory efforts may cause damage (termed patient self-inflicted lung injury) [28]. Although airway pressures during non-invasive ventilation were lower, our available data cannot discard an increased contribution of spontaneous breathing to these pressures, thus increasing transpulmonary pressures.
There is substantial debate on how different ventilatory settings are related to VILI. As previously described, tidal volume may promote regional overdistension. The use of reduced tidal volumes (6 ml/kg) decreased mortality in ARDS. Tidal volumes used in this CPE cohort are substantially higher than this value, and a threshold of 9 ml/kg has been correlated with a worse outcome [14]. From a pathogenetic point of view, tidal volume is a global measurement and local phenomena are driven by changes in pressure, which is sensed locally by lung cells. Hence, driving pressure, rather than tidal volume alone, has been proposed as a better marker of regional lung strain with better correlation to mortality than tidal volume in ARDS [29]. Respiratory system compliance, as a marker of the amount of lung available for ventilation, emerges then as a relevant biomarker to identify the risk of VILI. Our results, using a weighted marginal structural model that isolates the effects of driving pressures from other confounders, support the association between driving pressures and mortality in patients with CPE. On the other hand, PEEP, which is a major determinant of lung recruitment, may have multifaceted effects on VILI, as increasing end-expiratory volume may promote the recruitment of collapsed or flooded alveoli for ventilation, but also cause overdistension of previously aerated areas [30].
Heart–lung interactions are a major concern in mechanically ventilated patients [31]. Our data show progressively increased airway pressures according to the severity of the hemodynamic impairment. The increase in peak, plateau and driving pressures, with no change in tidal volume, can be explained by a progressive decrease in lung compliance. These results raise the hypothesis that clinicians set tidal volume to ensure ventilation, and the obtained pressures are the consequence of the magnitude of lung edema and its impact on respiratory mechanics. Regarding PEEP, that is usually set at lower levels in these patients due to its potential hemodynamic effects [13], no conclusion can be extracted.
This study has some limitations that must be discussed. Available data do not include information on previous cardiac diseases or triggering events. Similarly, the database did not differentiate between patients with preserved or reduced ejection fraction or other heart failure phenotypes [32, 33] although it has been reported that respiratory support is related to worse outcomes in both groups [34]. Therefore, we cannot discard differences in the observed effects of mechanical ventilation on the outcome among CPE phenotypes. Instead, the hemodynamic item of SOFA score was used to categorize the circulatory status at admission. It has been shown that SOFA score has a good prognostic value in patients with heart failure, independently of the baseline ejection fraction [35, 36]. In addition, the observational design allows only for associative conclusions, although the use of inverse probability of treatment weights in a marginal structural model increases the strength of this association by standardizing baseline risks [37]. Finally, plateau pressures were available in 75% of all patients. When a pressure control mode or non-invasive ventilation was registered, inspiratory pressure was considered as plateau pressure. However, excluding these patients from the analysis yielded the same results.
Conclusions
Our findings highlight the impact of mechanical ventilation in patients with CPE. Although the observational nature of this study prevents any causality relationship to be inferred, our results show that ventilatory variables could be used as a marker of severity and suggest that patients with CPE may be susceptible to VILI. Clinical trials of low tidal volume in CPE should test this hypothesis.
Availability of data and materials
Data access policy for LUNG SAFE study is available to researchers at https://www.esicm.org/trials-group-2-lung-safe/.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- COPD:
-
Chronic obstructive pulmonary disease
- CPE:
-
Cardiogenic pulmonary edema
- HR:
-
Hazard ratio
- PEEP:
-
Positive end-expiratory pressure
- SOFA:
-
Sequential organ failure assessment
- VILI:
-
Ventilator-induced lung injury
References
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD003844.pub4.
Vergani G, Cressoni M, Crimella F, L’Acqua C, Sisillo E, Gurgitano M, et al. A morphological and quantitative analysis of lung CT scan in patients with acute respiratory distress syndrome and in cardiogenic pulmonary edema. J Intensive Care Med. 2020;35:284–92.
Pham T, Pesenti A, Bellani G, Rubenfeld G, Fan E, Bugedo G, et al. Outcome of acute hypoxaemic respiratory failure: insights from the LUNG SAFE study. Eur Respir J. 2021;57:2003317.
Osman M, Syed M, Patibandla S, Sulaiman S, Kheiri B, Shah MK, et al. Fifteen-year trends in incidence of cardiogenic shock hospitalization and in-hospital mortality in the United States. J Am Heart Assoc. 2021;10: e021061.
Schrage B, Becher PM, Goßling A, Savarese G, Dabboura S, Yan I, et al. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail. 2021;8:1295–303.
Lesage A, Ramakers M, Daubin C, Verrier V, Beynier D, Charbonneau P, et al. Complicated acute myocardial infarction requiring mechanical ventilation in the intensive care unit: prognostic factors of clinical outcome in a series of 157 patients. Crit Care Med. 2004;32:100–5.
Kouraki K, Schneider S, Uebis R, Tebbe U, Klein HH, Janssens U, et al. Characteristics and clinical outcome of 458 patients with acute myocardial infarction requiring mechanical ventilation. Results of the BEAT registry of the ALKK-study group. Clin Res Cardiol Off J Ger Card Soc. 2011;100:235–9.
Miller PE, Van Diepen S, Metkus TS, Alviar CL, Rayner-Hartley E, Rathwell S, et al. Association between respiratory failure and clinical outcomes in patients with acute heart failure: analysis of 5 pooled clinical trials. J Card Fail. 2021;27:602–6.
Jentzer JC, Alviar CL, Miller PE, Metkus T, Bennett CE, Morrow DA, et al. Trends in therapy and outcomes associated with respiratory failure in patients admitted to the cardiac intensive care unit. J Intensive Care Med. 2022;37:543–54.
Metkus T, Miller PE, Alviar CL, Jentzer JC, van Diepen S, Katz JN, et al. Incidence, predictors and prognosis of respiratory support in non-ST segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2020. https://doi.org/10.1177/2048872620919947.
Nandiwada S, Islam S, Jentzer JC, Miller PE, Fordyce CB, Lawler P, et al. The association between cardiac intensive care unit mechanical ventilation volumes and in-hospital mortality. Eur Heart J Acute Cardiovasc Care. 2021;10:797–805.
Alviar CL, Miller PE, McAreavey D, Katz JN, Lee B, Moriyama B, et al. Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol. 2018;72:1532–53.
Shorofsky M, Jayaraman D, Lellouche F, Husa R, Lipes J. Mechanical ventilation with high tidal volume and associated mortality in the cardiac intensive care unit. Acute Card Care. 2014;16:9–14.
Yang Q, Zheng J, Chen X, Chen W, Wen D, Xiong X, et al. Relationship between driving pressure and mortality in ventilated patients with heart failure: a cohort study. Can Respir J. 2021;2021:5574963.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.
Therneau TM. A package for survival analysis in R. 2020. https://CRAN.R-project.org/package=survival. Accessed 6 June 2021.
van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. J Stat Softw. 2011;43:1–23.
Visser I, Speekenbrink M. depmixS4: an R package for hidden Markov models. J Stat Softw. 2010;36:1–21.
Wickham H. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York; 2016.
Tang Y, Horikoshi M, Li W. ggfortify: unified interface to visualize statistical result of popular R packages. R J. 2016;8:474–85.
Vallabhajosyula S, Kashani K, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, et al. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000–2014. Ann Intensive Care. 2019;9:96.
Aissaoui N, Puymirat E, Delmas C, Ortuno S, Durand E, Bataille V, et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail. 2020;22:664–72.
Writing Group for the PReVENT Investigators, Simonis FD, Serpa Neto A, Binnekade JM, Braber A, Bruin KCM, et al. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. JAMA. 2018;320:1872–80.
Amado-Rodríguez L, Del Busto C, López-Alonso I, Parra D, Mayordomo-Colunga J, Arias-Guillén M, et al. Biotrauma during ultra-low tidal volume ventilation and venoarterial extracorporeal membrane oxygenation in cardiogenic shock: a randomized crossover clinical trial. Ann Intensive Care. 2021;11:132.
Masip J, Roque M, Sanchez B, Fernandez R, Subirana M, Exposito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA. 2005;294:3124–30.
Hongisto M, Lassus J, Tarvasmaki T, Sionis A, Tolppanen H, Lindholm MG, et al. Use of noninvasive and invasive mechanical ventilation in cardiogenic shock: a prospective multicenter study. Int J Cardiol. 2017;230:191–7.
Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–42.
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55.
Garcia-Prieto E, Lopez-Aguilar J, Parra-Ruiz D, Amado-Rodriguez L, Lopez-Alonso I, Blazquez-Prieto J, et al. Impact of recruitment on static and dynamic lung strain in acute respiratory distress syndrome. Anesthesiology. 2016;124:443–52.
Monnet X, Teboul JL, Richard C. Cardiopulmonary interactions in patients with heart failure. Curr Opin Crit Care. 2007;13:6–11.
Chioncel O, Mebazaa A, Harjola V-P, Coats AJ, Piepoli MF, Crespo-Leiro MG, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:1242–54.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Metkus TS, Stephens RS, Schulman S, Hsu S, Morrow DA, Eid SM. Respiratory support in acute heart failure with preserved vs reduced ejection fraction. Clin Cardiol. 2020;43:320–8.
Elias A, Agbarieh R, Saliba W, Khoury J, Bahouth F, Nashashibi J, et al. SOFA score and short-term mortality in acute decompensated heart failure. Sci Rep. 2020;10:20802.
Aoyama D, Morishita T, Uzui H, Miyazaki S, Ishida K, Kaseno K, et al. Sequential organ failure assessment score on admission predicts long-term mortality in acute heart failure patients. ESC Heart Fail. 2020;7:244–52.
Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol Camb Mass. 2000;11:550–60.
Acknowledgements
See Additional file 1 for a list of LUNG SAFE collaborators.
Funding
Supported by Centro de Investigación Biomédica en Red (CIBER)-Enfermedades respiratorias, Madrid, Spain (CB17/06/00021) and Fundación para el Fomento en Asturias de la Investigación Científica aplicada y la tecnología (FICYT, AYUD2021/52014). RRG is the recipient of a grant from Instituto de Salud Carlos III, Madrid, Spain (CM20/00083).
Author information
Authors and Affiliations
Consortia
Contributions
Concept and design: LA-R, GAM. Acquisition, analysis and interpretation of the data: LAM, RR-G, GM, JGL, TP, EF, FM, GMA. Drafting of the manuscript: LA-R, GMA. Critical revision of the manuscript for important intellectual content: LA-R, RR-G, GB, JGL, TP, EF, FM, GMA. Statistical analysis: LA-R, GMA. Dr LA-R and Dr. GMA had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participating ICUs obtained ethics committee approval and obtained either patient consent or ethics committee waiver of consent (due to the observational nature of the study).
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Differences between patients who received non-invasive and invasive ventilation as first-line respiratory support. Table S2. Differences between patients with not prolonged and prolonged invasive mechanical ventilation (more than 10 days). Table S3. Hazard ratio for mortality of each variable included in the marginal structural model (n=248). Table S4. Hazard ratio for mortality of each variable included in a marginal structural model including only patients under controlled mechanical ventilation on ICU day 1 (N=209). Table S5. Hazard ratio for mortality of each variable included in a marginal structural model excluding patients in which plateau pressures were imputed (n= 167 patients). Table S6. Hazard ratio for mortality of each variable included in a marginal structural model excluding patients in which diagnosis of CPE is based only on clinical observations and not supported by any diagnostic technique (n= 229 patients). Table S7. Hazard ratio for mortality of each variable included in a marginal structural model using dynamic driving pressures (peak inspiratory pressure minus PEEP) (n=287). Table S8. Hazard ratio for mortality of each variable included in a marginal structural model including patients without invasive mechanical ventilation at ICU admission (n=295). Figure S1. Hazard ratios (HRs) and 95% confidence intervals (95% CI) of driving pressure obtained in different sensitivity analyses described in Tables S3–S7. CPE: Cardiogenic pulmonary edema.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amado-Rodríguez, L., Rodríguez-Garcia, R., Bellani, G. et al. Mechanical ventilation in patients with cardiogenic pulmonary edema: a sub-analysis of the LUNG SAFE study. j intensive care 10, 55 (2022). https://doi.org/10.1186/s40560-022-00648-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-022-00648-x