Abstract
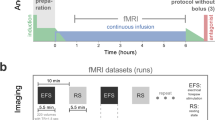
Deepening anesthesia produces well known changes in electroencephalogram (EEG) and evoked potentials, differing in pathological and normal brain. Yet, it is not known how the T2*-weighted signal changes in the healthy brain during deepening anesthesia. We studied the effect of thiopental bolus on functional magnetic resonance imaging (fMRI) in the healthy brain using porcine model. In five pigs (2–3 months, 20–25 kg), the control bolus prior to fMRI resulted in a change into burst-suppression. After the recovery of continuous EEG, fMRI (4 min) was performed with a single bolus of thiopental (11.4-17.1 mg/kg) administered 1 min after the onset of imaging. This was repeated in four of five pigs. Positive (6-8%) or negative (-3 to -8%) signal intensity changes correlated to the thiopental bolus injection were seen in the group average fMRI response. Positive response was 1.6% and negative response 2.3% of the total brain region of interest (ROI) voxels. Responding voxels were distributed more prominently in the thalamic ROI (4.5%) than in the cortical ROI (2.2%). The group average of unthresholded voxel time courses showed that the net effect of thiopental bolus was a small (0.5%) but a clear positive change in the thalamic region, while variance changed in the global level. In conclusion, this study is the first to show that significant signal intensity changes occur in fMRI response during the sudden deepening of thiopental anesthesia. However, these responses are neither anatomically constant nor global in the healthy swine brain.
Similar content being viewed by others
References
Liu J, Singh H, White PF. Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation. Anesth Analg 1997;84:185–9.
Sloan TB. Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol 1998;15:217–26.
Kassell NF, Hitchon PW, Gerk MK, Sokoll MD, Hill TR. Alterations in cerebral blood flow, oxygen metabolism, and electrical activity produced by high dose sodium thiopental. Neurosurgery 1980;7:598–602.
Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol 1994;90:l-16.
Hufnagel A, Burr W, Elger CE, Nadstawek J, Hefner G. Localization of the epileptic focus during methohexital-induced anesthesia. Epilepsia 1992;33:271–84.
Wennberg R, Quesney F, Olivier A, Dubeau F. Epileptiform and non-epileptiform paroxysmal activity from isolated cortex after functional hemisperectomy. Electroencephalogr Clin Neurophysiol 1997;102:437–42.
Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta 1982;714:265–70.
Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci 1990;87:9868–72.
Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci 1998;95:1834–9.
Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 1999;42:849–63.
Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the balloon model, Volterra kernels, and other hemodynamics. Neurolmage 2000; 12:466–77.
Zhang Z, Andersen AH, Avison MJ, Gerhardt GA, Gash DM. Functional MRI of apomorphine activation of the basal ganglia in awake rhesus monkeys. Brain Res 2000;852:290–6.
Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med 1999;41:412–6.
Martin E, Thiel T, Joeri P, Loenneker T, Ekatodramis D, Huisman T, Hennig J, Marcar VL. Effect of pentobarbital on visual processing in man. Hum Brain Mapp 2000;10:132–9.
Kiviniemi V, Jauhiainen J, Tervonen O, PÄÄkkö E, Oikarinen J, VainionpÄÄ V, Rantala H, Biswal B. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med 2000;44:373–8.
Ives JR, Warach S, Schmitt F, Edelman RR, Schomer DL. Monitoring the patient’s EEG during echo planar MRI. Electroencephalogr Clin Neurophysiol 1993;87:417–20.
Moser E, Teichtmeister C, Diemling M. Reproducibility and postprocessing of gradient-echo functional MRI to improve localization of brain activity in the human visual cortex. Magn Reson Imaging 1996;14:567–79.
Zaharchuk G, Mandeville JB, Bogdanov AA, Weissleder R, Rosen BR, Marota JJA. Cerebrovascular dynamics of autoregulation and hypoperfusion. An MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke 1999;30:2197–205.
Kalisch R, Elbel GK, Gossl C, Czisch M, Auer DP. Blood pressure changes induced by arterial blood withdrawal influence bold signal in anesthesized rats at 7 tesla: implications for pharmacologic MRI. Neuroimage 2001;14:891–998.
Bührer M, Maitre PO, Hung OR, Ebling WF, Shafer SL, Stanski DR. Thiopental pharmacodynamics. Anesthesiology 1992;77:226–36.
Maclver MB, Mandema JW, Stanski DR, Bland BH. Thiopental uncouples hippocampal and cortical synchronized electroencephalographic activity. Anesthesiology 1996;84:1411–24.
Pierce EC, Lambertsen CJ, Deutsch S, Chase PE, Linde HW, Dripps RD, Price HL. Cerebral circulation and metabolism during thiopental anesthesia and hyperventilation in man. J Clin Invest 1962;40:1664–71.
Stullken EH, Milde JH, Michenfelder JD, Tinker JH. The nonlinear responses of cerebral metabolism to low concentrations of halothane, enflurane, isoflurane, and thiopental. Anesthesiology 1977;46:28–34.
Björkman S, Nilsson F, åkeson J, Messeter K, Rosén I. The effect of thiopental on cerebral blood flow, and its relation to plasma concentration, during simulated induction of anaesthesia in a porcine model. Acta Anaesthesiol Scand 1994;38:473–8.
Prys-Roberts C. Cardiovascular and ventilatory effects of intravenous anesthetics. Clin Anaesth 1984;2:203–21.
Sokoloff L. In: Greger R, Windhorst U, editors. Circulation in the Central Nervous System in Comprehensive Human Physiology, vol. 1. Berlin: Springer-Verlag, 1996:568.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedures, and normal values in the consious and anesthetized albino rat. J Neurochem 1977;28:897–916.
Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg 1999;88:554–8.
Levin JM, Frederick Bde B, Ross MH, Fox JF, von Rosenberg HL, Kaufman MJ, Lange N, Mendelson JH, Cohen BM, Renshaw PF. Influence of baseline hematocrit and hemodilution on BOLD fMRI activation. Magn Reson Imaging 2001;19(8):1055–62.
Anttila V, RimpilÄinen J, Pokela M, Kiviluoma K, MÄkiranta M, JÄntti V, VainionpÄÄ V, Hirvonen J, Juvonen T. Lamotrigine improves cerebral outcome after hypothermic circulatory arrest: a study in a chronic porcine model. J Thorac Cardiovasc Surg 2000;120:247–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MÄkiranta, M.J., Jauhiainen, J.P.T., Oikarinen, J.T. et al. Functional magnetic resonance imaging of swine brain during change in thiopental anesthesia into EEG burst-suppression level — A preliminary study. MAGMA 15, 27–35 (2002). https://doi.org/10.1007/BF02693841
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02693841