Abstract
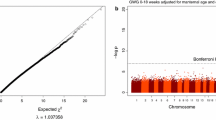
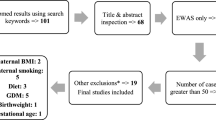
This study aims to evaluate the association of maternal DNA methylation (DNAm) during pregnancy and offspring birthweight. One hundred twenty-two newborn-mother dyads from the Isle of Wight (IOW) cohort were studied to identify differentially methylated cytosine-phosphate-guanine sites (CpGs) in maternal blood associated with offspring birthweight. Peripheral blood samples were drawn from mothers at 22–38 weeks of pregnancy for epigenome-wide DNAm assessment using the Illumina Infinium HumanMethylation450K array. Candidate CpGs were identified using a course of 100 repetitions of a training and testing process with robust regressions. CpGs were considered informative if they showed statistical significance in at least 80% of training and testing samples. Linear mixed models adjusting for covariates were applied to further assess the selected CpGs. The Swedish Born Into Life cohort was used to replicate our findings (n = 33). Eight candidate CpGs corresponding to the genes LMF1, KIF9, KLHL18, DAB1, VAX2, CD207, SCT, SCYL2, DEPDC4, NECAP1, and SFRS3 in mothers were identified as statistically significantly associated with their children’s birthweight in the IOW cohort and confirmed by linear mixed models after adjusting for covariates. Of these, in the replication cohort, three CpGs (cg01816814, cg23153661, and cg17722033 with p values = 0.06, 0.175, and 0.166, respectively) associated with four genes (LMF1, VAX2, CD207, and NECAP1) were marginally significant. Biological pathway analyses of three of the genes revealed cellular processes such as endocytosis (possibly sustaining an adequate maternal-fetal interface) and metabolic processes such as regulation of lipoprotein lipase activity (involved in providing substrates for the developing fetus). Our results contribute to an epigenetic understanding of maternal involvement in offspring birthweight. Measuring DNAm levels of maternal CpGs may in the future serve as a diagnostic tool recognizing mothers at risk for pregnancies ending with altered birthweights.

Similar content being viewed by others
Data Availability
Data used in this study may be available upon request from the authors with permission from the IOW and Born Into Life cohorts.
References
Asmare G, Berhan N, Berhanu M, Alebel A. Determinants of low birth weight among neonates born in Amhara Regional State Referral Hospitals of Ethiopia: unmatched case control study. BMC Res Notes. 2018;11(1):447. https://doi.org/10.1186/s13104-018-3568-2.
Xu XF, Li YJ, Sheng YJ, Liu JL, Tang LF, Chen ZM. Effect of low birth weight on childhood asthma: a meta-analysis. BMC Pediatr. 2014;14:275. https://doi.org/10.1186/1471-2431-14-275.
Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91(4):334–9. https://doi.org/10.1136/adc.2005.080390.
Palatianou ME, Simos YV, Andronikou SK, Kiortsis DN. Long-term metabolic effects of high birth weight: a critical review of the literature. Horm Metab Res. 2014;46(13):911–20. https://doi.org/10.1055/s-0034-1395561.
Kupers LK, Monnereau C, Sharp GC, Yousefi P, Salas LA, Ghantous A, et al. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat Commun. 2019;10(1):1893. https://doi.org/10.1038/s41467-019-09671-3.
Kwon EJ, Kim YJ. What is fetal programming?: a lifetime health is under the control of in utero health. Obstet Gynecol Sci. 2017;60(6):506–19. https://doi.org/10.5468/ogs.2017.60.6.506.
Chen S, Mukherjee N, Janjanam VD, Arshad SH, Kurukulaaratchy RJ, Holloway JW, et al. Consistency and Variability of DNA Methylation in Women During Puberty, Young Adulthood, and Pregnancy. Genet Epigenet. 2017;9:1179237x17721540. https://doi.org/10.1177/1179237x17721540.
Arshad SH, Holloway JW, Karmaus W, Zhang H, Ewart S, Mansfield L, et al. Cohort Profile: The Isle Of Wight Whole Population Birth Cohort (IOWBC). Int J Epidemiol. 2018;47(4):1043–i. https://doi.org/10.1093/ije/dyy023.
Ogbuanu IU, Karmaus W, Arshad SH, Kurukulaaratchy RJ, Ewart S. Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax. 2009;64(1):62–6. https://doi.org/10.1136/thx.2008.101543.
Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics (Oxford, England). 2014;30(10):1363–9. https://doi.org/10.1093/bioinformatics/btu049.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. https://doi.org/10.1186/1471-2105-13-86.
Smew AI, Hedman AM, Chiesa F, Ullemar V, Andolf E, Pershagen G, et al. Limited association between markers of stress during pregnancy and fetal growth in 'Born into Life', a new prospective birth cohort. Acta Paediatr. 2018;107(6):1003–10. https://doi.org/10.1111/apa.14246.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. https://doi.org/10.1093/nar/16.3.1215.
Bibikova M, Fan JB. GoldenGate assay for DNA methylation profiling. Methods Mol Biol. 2009;507:149–63. https://doi.org/10.1007/978-1-59745-522-0_12.
Wang D, Yan L, Hu Q, Sucheston LE, Higgins MJ, Ambrosone CB, et al. IMA: an R package for high-throughput analysis of Illumina's 450K Infinium methylation data. Bioinformatics. 2012;28(5):729–30. https://doi.org/10.1093/bioinformatics/bts013.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–27. https://doi.org/10.1093/biostatistics/kxj037.
Du P, Zhang X, Huang CC, Jafari N, Kibbe WA, Hou L, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. https://doi.org/10.1186/1471-2105-11-587.
Ray MA, Tong X, Lockett GA, Zhang H, Karmaus WJ. An efficient approach to screening Epigenome-wide data. Biomed Res Int. 2016;2016:2615348–16. https://doi.org/10.1155/2016/2615348.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
Welch RP, Willer CJ, J. SL, Boehnke M. Snipper: a research tool for extracting and searching biological annotations on genes near SNPs. University of Michigan; 2013.
Casper J, Zweig AS, Villarreal C, Tyner C, Speir ML, Rosenbloom KR, et al. The UCSC Genome Browser database: 2018 update. Nucleic Acids Res. 2018;46(D1):D762–d9. https://doi.org/10.1093/nar/gkx1020.
Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37(Web Server issue):W305–11. https://doi.org/10.1093/nar/gkp427.
Feinberg H, Taylor ME, Razi N, McBride R, Knirel YA, Graham SA, et al. Structural basis for langerin recognition of diverse pathogen and mammalian glycans through a single binding site. J Mol Biol. 2011;405(4):1027–39. https://doi.org/10.1016/j.jmb.2010.11.039.
Feinberg H, Rowntree TJ, Tan SL, Drickamer K, Weis WI, Taylor ME. Common polymorphisms in human langerin change specificity for glycan ligands. J Biol Chem. 2013;288(52):36762–71. https://doi.org/10.1074/jbc.M113.528000.
Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12(1):71–81. https://doi.org/10.1016/s1074-7613(00)80160-0.
Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. https://doi.org/10.1146/annurev.immunol.22.012703.104558.
Blois SM, Kammerer U, Alba Soto C, Tometten MC, Shaikly V, Barrientos G, et al. Dendritic cells: key to fetal tolerance? Biol Reprod. 2007;77(4):590–8. https://doi.org/10.1095/biolreprod.107.060632.
Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019;4(31). https://doi.org/10.1126/sciimmunol.aat6114.
Lamb CA, Dooley HC, Tooze SA. Endocytosis and autophagy: shared machinery for degradation. Bioessays. 2013;35(1):34–45. https://doi.org/10.1002/bies.201200130.
Kanninen TT, de Andrade Ramos BR, Witkin SS. The role of autophagy in reproduction from gametogenesis to parturition. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):3–8. https://doi.org/10.1016/j.ejogrb.2013.07.020.
Cao B, Camden AJ, Parnell LA, Mysorekar IU. Autophagy regulation of physiological and pathological processes in the female reproductive tract. Am J Reprod Immunol. 2017;77(5). https://doi.org/10.1111/aji.12650.
Yang S, Wang H, Li D, Li M. Role of endometrial autophagy in physiological and pathophysiological processes. J Cancer. 2019;10(15):3459–71. https://doi.org/10.7150/jca.31742.
Peterfy M. Lipase maturation factor 1: a lipase chaperone involved in lipid metabolism. Biochim Biophys Acta. 2012;1821(5):790–4. https://doi.org/10.1016/j.bbalip.2011.10.006.
Ehrhardt N, Bedoya C, Peterfy M. Embryonic viability, lipase deficiency, hypertriglyceridemia and neonatal lethality in a novel LMF1-deficient mouse model. Nutr Metab (Lond). 2014;11:37. https://doi.org/10.1186/1743-7075-11-37.
Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol. 2014;15(1):24–31. https://doi.org/10.2174/1389201015666140330192345.
Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002;19(1):43–55. https://doi.org/10.1385/endo:19:1:43.
Heerwagen MJR, Gumina DL, Hernandez TL, Van Pelt RE, Kramer AW, Janssen RC, et al. Placental lipoprotein lipase activity is positively associated with newborn adiposity. Placenta. 2018;64:53–60. https://doi.org/10.1016/j.placenta.2018.03.001.
Segura MT, Demmelmair H, Krauss-Etschmann S, Nathan P, Dehmel S, Padilla MC, et al. Maternal BMI and gestational diabetes alter placental lipid transporters and fatty acid composition. Placenta. 2017;57:144–51. https://doi.org/10.1016/j.placenta.2017.07.001.
Gagne-Ouellet V, Houde AA, Guay SP, Perron P, Gaudet D, Guerin R, et al. Placental lipoprotein lipase DNA methylation alterations are associated with gestational diabetes and body composition at 5 years of age. Epigenetics. 2017;12(8):616–25. https://doi.org/10.1080/15592294.2017.1322254.
Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation--review. Placenta. 2010;31(10):839–47. https://doi.org/10.1016/j.placenta.2010.07.011.
Tepekoy F, Akkoyunlu G, Demir R. The role of Wnt signaling members in the uterus and embryo during pre-implantation and implantation. J Assist Reprod Genet. 2015;32(3):337–46. https://doi.org/10.1007/s10815-014-0409-7.
Dunk C, Kwan M, Hazan A, Walker S, Wright JK, Harris LK, et al. Failure of decidualization and maternal immune tolerance underlies uterovascular resistance in intra uterine growth restriction. Front Endocrinol. 2019;10:160. https://doi.org/10.3389/fendo.2019.00160.
Huang Z, Ge YF, Jing J, Wu L, Zhou ZY, Zhu QF, et al. Effect of secretin on the expression of cPLA2 and mPGEs-1 in mouse endometrial stromal cell during early pregnancy. Sheng Li Xue Bao. 2016;68(6):725–32.
Huang Z, Wang TS, Qi QR, Zuo RJ, Liang XH, Zhao XY, et al. Progesterone regulates secretin expression in mouse uterus during early pregnancy. Reprod Sci. 2014;21(6):724–32. https://doi.org/10.1177/1933719113512527.
Knox K, Leuenberger D, Penn AA, Baker JC. Global hormone profiling of murine placenta reveals secretin expression. Placenta. 2011;32(11):811–6. https://doi.org/10.1016/j.placenta.2011.08.013.
Siu FK, Sham MH, Chow BK. The prenatal expression of secretin receptor. Ann N Y Acad Sci. 2006;1070:561–5. https://doi.org/10.1196/annals.1317.081.
Kramer MS. The epidemiology of low birthweight. Nestle Nutrition Institute workshop series. 2013;74:1–10. https://doi.org/10.1159/000348382.
Pan H, Holbrook JD, Karnani N, Kwoh CK. Gene, Environment and Methylation (GEM): a tool suite to efficiently navigate large scale epigenome wide association studies and integrate genotype and interaction between genotype and environment. BMC Bioinformatics. 2016;17:299. https://doi.org/10.1186/s12859-016-1161-z.
Funding
This work has been supported by National Institute of Allergy and Infectious Diseases [R01 AI091905] and the National Heart, Lung, and Blood Institute [R01 HL132321] to W.K and [R01 HL082925] to SHA. The Born Into Life cohort was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Swedish Heart-Lung Foundation and Department of Clinical Sciences, Karolinska Institutet, Danderyd University Hospital, Stockholm, Sweden. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
The IOW study was approved repeatedly by the local research ethics committee (NRES Committee South Central—Hampshire B, UK) and the University of Memphis Institutional Review Board in Memphis, USA (FWA00006815). Ethical approval was obtained by the Regional Ethics Review Board in Stockholm, Sweden, for the Born Into Life study.
Consent to Participate and for Publication
Written consents were obtained from all participants of the IOW and Born Into Life cohorts at recruitment and follow-ups.
Code Availability
N/A
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary fig. 1
Structural equation modeling graphs showing the total effect of maternal DNAm on birthweight, indirect effect of maternal DNAm on birthweight through gestational age, and the direct effects between maternal DNAm, gestational age, and birthweight. Models are adjusted for newborn’s gender, maternal peripheral blood cell types, maternal BMI, smoking during pregnancy, and socioeconomic status. Results are shown as beta values*100 (percentage) *0.001 ≤ P value < 0.05, **P value < 0.001(PUB 174 kb)
Rights and permissions
About this article
Cite this article
Kheirkhah Rahimabad, P., Arshad, S.H., Holloway, J.W. et al. Association of Maternal DNA Methylation and Offspring Birthweight. Reprod. Sci. 28, 218–227 (2021). https://doi.org/10.1007/s43032-020-00281-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00281-9