Abstract
Investigating resource partitioning of marine predators is essential for understanding coexistence of sympatric species and the functional role they play in marine ecosystems. Baleen whales are a key component of sub-Antarctic ecosystems, foraging predominantly on zooplankton and small forage fish. During the twentieth century, baleen whales were unsustainably exploited across the Southern Ocean. Within the exclusive economic zone of South Georgia and the South Sandwich Islands (SGSSI EEZ) in the South Atlantic, approximately 98,000 fin whales (Balaenoptera physalus) and 16,000 sei whales (B.borealis) were harvested. Despite both species historically occurring in high numbers and feeding in sub-polar waters, little is known about the mechanisms of coexistence. Here, by measuring stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) in archived baleen plates and analysing historic catch data, we investigate resource partitioning of fin and sei whale during the commercial whaling period. Temporal and spatial occupancy at SGSSI EEZ (inferred from whaling catches that occurred between 1904 and 1976), alongside historic stomach contents (from the literature), and δ13C and δ15N results (observed in this study), suggests that despite using a common prey resource there was limited overlap in isotopic niches between the two species, with sei whales using SGSSI waters later in the season and for a shorter period than fin whales. We hypothesise that the isotopic differences were most likely due to sei whales foraging at lower latitudes prior to arrival at SGSSI. Our data provide novel insight into how two sympatric whale species co-occurring at SGSSI during the commercial whaling period may have partitioned resources and provide a potential ecological baseline to assess changes in resource use in recovering whale populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resource partitioning is a mechanism that facilitates the coexistence of sympatric species within an ecosystem (Levin 2000; Roques and Chekroun 2011; Friedlaender et al. 2021). This mechanism, commonly referred to as the competitive exclusion principle, states that two species cannot occupy exactly the same niche and stably coexist (Hardin 1960), with “niche” previously defined as the ecological space where a species or population can persist and thus utilise resources and impact its environment (Polechová and Storch 2019) (although other similar definitions exist e.g. Grinnell 1924; Leibold 1995). Instead, to reduce competition sympatric species may target different prey taxa, different size classes of the same prey taxa, or target the same prey taxa at different times of day (or year), in different locations, or both (spatiotemporal partitioning) (Garneau et al. 2007; Gerrish and Morin 2016; Fossette et al. 2017; Morera-Pujol et al. 2018; Gulka et al. 2019; Lea et al. 2020; Teixeira et al. 2021; Durante et al. 2021; Friedlaender et al. 2021). For example, whale species foraging on Antarctic krill near the Antarctic Peninsula between summer and autumn appear to differentiate krill prey by size class with humpback, Antarctic minke, and fin whales targeting small (< 35 mm), intermediate (35–44 mm), and large (> 45 mm) krill, respectively (Santora et al. 2010). In contrast, bull sharks and Atlantic tarpon in the tropical North Atlantic target the same prey groups within the same region at different times of the year (Hammerschlag et al. 2012), whilst blue and fin whales in the eastern North Pacific forage on similar prey over the same time period at different depths (Friedlaender et al. 2015). Ecosystem-based management can only be implemented effectively once the mechanisms facilitating resource partitioning and coexistence of marine predators on feeding grounds are known (e.g. fisheries closures coordinating with marine mammal occurrence and life history strategies to prevent accidental bycatch O’Keefe et al. 2013; Hoos et al. 2019)).
Understanding resource partitioning of Mysticeti (baleen whales) is important as they are top marine predators that provide essential ecosystem services, including the transfer of nutrients between the many habitats and regions of which they occur (Roman et al. 2014; Doughty et al. 2016). Moreover, engulfment foraging combined with large body size gives baleen whales the capacity to disproportionately impact ecosystem functioning relative to other top marine predators (Doughty et al. 2016; Goldbogen et al. 2017). Due to their large energetic requirements, baleen whales are often associated with highly productive areas of the ocean where they can target dense aggregations of prey (Kelt and Van Vuren 1999; Goldbogen et al. 2017; Kahane-Rapport et al. 2020; Cade et al. 2021a, b). These ephemeral prey hotspots (densely packed high-quality and often sporadic prey swarms) often facilitate resource competition by enticing multiple species and large numbers of baleen whales to forage in close proximity (Findlay et al. 2017; Cade 2021a; Rockwood et al. 2020; Cade et al. 2021b). However, the mechanisms driving the coexistence of mixed species aggregations of baleen whales are relatively understudied compared with other taxonomic groups, including but not exclusive to, seabirds (Robertson et al. 2014; Navarro et al. 2015; Pontón-Cevallos et al. 2017; Gulka et al. 2019; Reisinger et al. 2020; Mills et al. 2021) and marine fishes (Targett 1978; Ross 1986; Fanelli et al. 2011; Cherel et al. 2011; Lopez-Lopez et al. 2011; Sheaves et al. 2013; Pardo et al. 2015; Drago et al. 2017). Recent publications have demonstrated resource partitioning amongst baleen whales (McCarthy et al. 2021; García-Vernet et al. 2021). Baleen whale species have been observed foraging in common areas at different trophic levels (Gavrilchuk et al. 2014; Witteveen and Wynne 2016; Herr et al. 2016), whilst, at one well-documented feeding site, different species partition the same prey taxa by size class, depth, and distance to shore (Friedlaender et al. 2009, 2021). It is evident that whales can use a variety of mechanisms to facilitate sympatry; understanding these is important to identify foodweb dependencies and develop effective ecosystem-based management.
In the Southern Hemisphere, multiple species of baleen whales migrate to highly productive Antarctic and sub-Antarctic waters during the austral summer to feed (Mackintosh and Wheeler 1929; Mackintosh 1946; Nemoto 1962; Kawamura 1980), facilitating the potential for resource partitioning. The high latitudes of the South Atlantic represent some of the most productive and biodiverse areas in the Southern Ocean (Atkinson et al. 2001; Trathan et al. 2007, 2014) and baleen whales form a key component of marine predator guilds here (Brown 1968; Headland 1992; Zerbini et al. 2006; Kennedy et al. 2020). Marine resources around South Georgia and the South Sandwich Islands (SGSSI) in the South Atlantic are monitored and managed by the government of SGSSI within the boundaries of the SGSSI exclusive economic zone (EEZ). Since twentieth-century whaling ceased in 1986 (Rocha et al. 2015), there is now evidence of whale population size increases at SGSSI (Kennedy et al. 2020; Jackson et al. 2020; Baines et al. 2021). Of the five species that currently forage at SGSSI, or were observed foraging at SGSSI during the twentieth-century whaling period (blue whale, Balaenoptera musculus, fin whale, Balaenoptera physalus, humpback whale, Megaptera novaeangliae, Antarctic minke whale, Balaenoptera bonaerensis and sei whale, Balaenoptera borealis), two species (fin and sei whale) occurred sympatrically in sub-Antarctic waters during the whaling period, foraging primarily on zooplankton between spring and autumn (Kawamura 1980; Allison 2016). Although present-day diets of fin and sei whales at SGSSI remain unknown, historic stomach contents analyses indicate that fin and sei whales both consumed Antarctic krill (Euphausia superba) within SGSSI waters during the commercial whaling period (Matthews 1938; Brown 1968). Despite sharing a common prey resource (at least during the twentieth century), the mechanisms facilitating the coexistence of fin and sei whale consumption of Antarctic krill at SGSSI during the twentieth century and present day are unknown.
Historically, resource use has been investigated using stomach contents analysis; however, this approach is limited to a single point in time and will ultimately reflect prey availability at the time of the whale’s capture; therefore, studies using stomach contents may only provide partial information on diet (Votier et al. 2003; Bowen and Iverson 2012). In contrast, stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) provide a powerful alternative to stomach contents analysis, as they reflect prey consumption during the period of tissue synthesis. However, stable isotope analysis of animal tissues does not often provide information on specific prey species, although it can provide relative information on the resource use of marine predators within an ecosystem.
Stable isotope analysis has been widely used to study animal diets in the wild (Hobson 1999; Rubenstein and Hobson 2004; West et al. 2006), including baleen whales (e.g. Newsome et al. 2010a; Borrell et al. 2012; Eisenmann et al. 2016; Valenzuela et al. 2018; Reiss et al. 2020)), with a particular focus on carbon and nitrogen stable isotope ratios. As energy flows through an ecosystem and is passed up the food chain, carbon isotopic values (δ13C) can be used to determine the source of primary production within a food web (Fry 1989). As these sources differ somewhat systematically between habitats and regions, carbon isotopic values can provide a proxy for: (i) latitudinal distributions, with lower δ13C towards the polar regions; (ii) foraging distance from shore, as inshore benthic food webs differ predictably in baseline δ13C due to their detrital origin compared with offshore pelagic food webs; and (iii) foraging in deeper waters, with higher δ13C values at increasing depth (Francois et al. 1993; Hobson et al. 1994; Cherel and Hobson 2007; Linnebjerg et al. 2013; Espinasse et al. 2019; Michel et al. 2020). Nitrogen isotopic values (δ15N) provide information on trophic positioning with higher δ15N ratios occurring predictably up the food web (Schoeninger and DeNiro 1984; Wada et al. 1991; Boecklen et al. 2011; Healy et al. 2017), although the underlying physiological mechanisms driving this pattern are currently not fully understood (O’Connell 2017).
To better understand changes in species diet and resource use over time, historic baseline information on diet and resource use (often inferred from isotopic analysis) can be included to identify species responses to ongoing anthropogenic impacts and global environmental change (Visser and Both 2005; McClenachan et al. 2012; Clavero 2014). Isotopic information is retained in tissues post-mortem, with patterns stable over millennia, enabling the use of historic specimens to infer the resource use patterns of populations in the past (e.g. Zenteno et al. 2015). Baleen whales have multiple layers of tightly packed keratinous plates (baleen) surrounding the inner mouth edge that grow continuously from the gumline and are metabolically inert once synthesised (Rita et al. 2019) making baleen a good candidate tissue for studying temporal changes of baleen whale resource use. Indeed, isotopic analysis of incremental samples along the growth axis of baleen plates has been used to determine changes in whale foraging preferences through time (Best and Schell 1996; Trueman et al. 2019; Reiss et al. 2020).
To better understand habitat and resource use of wild populations, stable isotopes are often paired with spatiotemporal occurrence data (e.g. Robillard et al. 2021; Leal and Bugoni 2021). Historic information on the distribution of whale species can be inferred from the timing and location of twentieth-century whaling catches (e.g. Clapham et al. 2004; Reeves et al. 2004). Shore-based whaling occurred at the island of South Georgia (within the SGSSI management area) between 1904 and 1965 and pelagic boat-based whaling started in the 1920s and continued until 1976 (Tønnessen and Johnsen 1982; Headland 1992; Allison 2016). South Georgia has been described as an epicentre of twentieth-century whaling (Jackson et al. 2020), with 8.6% of the 2.1 million whales harvested across the Southern Hemisphere taken from this region (Allison 2016). Whaling catch records are available for approximately 98,000 fin whales and 16,000 sei whales within the SGSSI EEZ (Allison 2016). Present-day population size and levels of population recovery for fin and sei whales are currently unknown in the South Atlantic, and very little ecological data from present-day populations are available.
Here, using temporal and spatial information on fin and sei whale distribution from historic whaling catches, alongside, stable isotope analysis of incremental samples from six keratinous baleen plates collected from unique individuals and discarded during the twentieth-century whaling period, we investigate resource use and partitioning of fin whales and sei whales at SGSSI during the commercial whaling period. This information may help to infer the ecological role of fin and sei whales at SGSSI and identify evidence for interspecific resource partitioning.
Materials and methods
Spatial and temporal co-occurrence inferred from whaling catches at SGSSI
Whaling catches between 1904 and 1976 were obtained from the IWC catch database version 6.1 (Allison 2016) and used to provide information on the location of species-specific catches within the boundaries of the SGSSI EEZ. Spatiotemporal correlations in the locality and timing of whaling catches of fin and sei whales were used to investigate interspecific differences in spatial and temporal use of the marine environment around SGSSI.
Stable isotope analyses
Baleen plates (fin whales, n = 3; Sei whales, n = 3) were collected by individual researchers between 1970 and 1974 from the shorelines of Grytviken whaling station, South Georgia (54.3° S, 36.5° W), and samples were stored at room temperature prior to sampling. Plates were collected prior to international enforcement of regulations by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) in 1975; no CITES permits are associated with these materials. Baleen was subsampled using a 10 mm metal revolving belt punch from the part closest to the gum line, subsamples cleaned using a steel wool pad and 100% ethanol, and DNA extracted following the extraction protocol by Dabney et al. (2013) in the ancient DNA laboratory at the University of Cambridge (Dept. of Archaeology). Mitochondrial control region sequences were amplified using PCR and sequences compared against NCBI reference sequences to identify species. All mitochondrial haplotypes were unique to each baleen plate, thereby verifying that each belonged to a unique individual. PCR primers and amplification details are presented in supplementary Table S1.
Baleen growth rates were reviewed in the literature to ensure that, where possible, baleen samples were representative of a minimum period of one year [> 18 cm for fin whales (Bentaleb et al. 2011), > 16 cm for sei whales (Reiss et al. 2020)]. Plates were sampled incrementally along the longest axis every 1 cm using a metal revolving belt punch with 5 mm diameter. In total, 57 incremental samples were taken from sei whales (n = 3 plates, with 18, 19 and 20 increments per plate) and 54 from fin whales (n = 3 plates, with 15, 19 and 20 increments per plate).
Lipids were removed by alternating 2:1 chloroform/methanol mixture and 2:1 methanol/chloroform mixture whilst soaking in an ultrasonic bath, changing the solvent every 30 min until the solution remained clear; the number of washes varied across increments (range: 1–3). Carbonates were removed through demineralization in 0.5 M aq. hydrochloric acid and extracts rinsed using triplicate washes of deionised H2O. Samples were then freeze-dried and weighed (mean ± SD, 0.8 ± 0.1 mg) in triplicate into tin capsules. Stable isotope ratios of carbon and nitrogen were measured using a Costech Elemental Analyser coupled with a Thermo Delta V Plus continuous flow stable isotope mass spectrometer (EA-IRMS), at the Godwin Laboratory, Department of Earth Sciences, University of Cambridge. Replicate analyses of international standards were used to correct for instrumental drift. Carbon and nitrogen stable isotope values are expressed as delta values (δ) relative to international standards (Vienna PeeDee Belemnite—δ13C and atmospheric N2—δ15N; see (Hoefs 2018)). Repeated measurements of in-house standards (caffeine, nylon, alanine, protein 2) were used to determine measurement precision (δ13C—0.1, δ15N—0.2).
Mass spectrometry results were retained for analysis if they passed the following quality control (QC) checks: (i) atomic carbon/nitrogen ratio (C:N hereafter) remained at approximately 3.4 (± 0.5) (see: O’Connell and Hedges 1999; O’Connell et al. 2001; Newsome et al. 2010b; Bentaleb et al. 2011; Ryan et al. 2013), and (ii) lipids were removed successfully (accidental retainment of lipid was assessed using correlation between δ13C and C:N (Post et al. 2007)). Triplicate repeats of incremental δ13C and δ15N were averaged prior to data analysis.
Data analysis
All analyses were performed using software R version 4.0 (RStudio Team 2020), and maps created using open-source geospatial software, qGIS version 3.10.13 (Quantum 2017).
Spatial and temporal co-occurrence inferred from whaling catches at SGSSI
Spatial patterns
To enable comparison of species-specific distributions whilst accounting for interspecific differences in the relative abundance inferred from catch data, whaling catch densities were mapped into 72 equal sized hexagonal bins and percentiles calculated using the quantile function in R (version 4.0). Each hexagonal bin spanned two decimal degrees longitude and latitude across the SGSSI EEZ, and catches were summed within each bin. A correlation coefficient of spatial association (Clifford et al. 1989; Dutilleul et al. 1993) was used to infer similarities in distribution (spatial partitioning) between fin and sei whales within the SGSSI EEZ using the “modified.ttest” function in the package SpatialPack (Osorio et al. 2014). This coefficient measures the amount of correlation between two spatial sequences (in this case, the distribution of whaling catches of fin and sei whales), with values close to 0 representative of no spatial correlation and values close to 1 representative of complete spatial correlation.
Temporal patterns
Monthly catch rates were calculated for each species (total catches that occurred within the SGSSI EEZ between 1904 and 1976 per month) and the similarity in the peak timing of catches was compared using a cross-correlation analysis. Cross-correlation measures the similarity of two time series, whereby two identical (or almost identical time series) will display a peak at zero (Derrick and Thomas 2004). Cross-correlations were performed using the “ccf” function in the `base` stats package in R and a significant correlation assessed using critical values at the 5% level (R Development Core Team 2003); a significant value of + 1 or higher would resemble significant lag in the peak timing of catches of one or two months, respectively.
To identify whether variation in whaler behaviour throughout the commercial whaling period [e.g. whalers foraging inshore or offshore; whaler preference for a particular species at a given time (fin whales: 1937–1965; sei whales: 1965–1975)] may have resulted in spatiotemporal variation in the occurrence of fin and sei whales at SGSSI, catch data were binned into three time periods (early: 1904–1928; middle: 1929–1952; late: 1953–1976). We then compared interspecific differences in temporal (cross-correlations) and spatial (modified t tests) catch patterns within and between each time period analysed.
Measuring historic resource partitioning using stable isotope analysis
Interspecific variation
The Mann–Whitney U test (MacFarland and Yates 2016) was used to identify statistical differences in δ13C and δ15N values between fin and sei whales as inference of variation in resource use between these two species. A nonparametric test was used as the assumptions of residual normality and homogeneity of variance were violated (Shapiro Wilk’s and Levene’s test, respectively).
The ecological niche space of fin whale and sei whale was inferred from pairwise values of δ13C and δ15N from baleen plates under a bivariate normal distribution (ellipses of multivariate δ-space are commonly used for ecological applications (Newsome and Martinez del Rio 2007; Newsome et al. 2010a, b). Multivariate δ-space of fin whale and sei whale at SGSSI was estimated using two models: (i) using maximum likelihood estimates corrected for small sample sizes (SEAC) and (ii) using Bayesian estimates of isotopic niche space (SEAB), both calculated using the R package SIBER (Jackson et al. 2011). To identify isotopic evidence for resource partitioning, percentage overlap of niche space between fin whales and sei whales was measured using the bayesianOverlap function from the R package SIBER (Jackson et al. 2011). Niche size and overlap were measured twice for both models (SEAC and SEAB). First, only incorporating c.40% (reflecting the core trophic niche) and second using c.95% (reflecting the full trophic niche), following Jones et al. (2020). SIBER uses a Markov chain Monte Carlo (MCMC) model-fitting algorithm. MCMC parameters were as follows: 100,000 iterations, 1000 burn-in, 5% thinning and 3 independent chains. Coherence among chains were determined for each model parameter using a Gelman-Rubin diagnostics of < 1.1 (Gelman and Rubin 1992; Brooks and Gelman 1998). Species differences in SEAB were compared by assessing overlap of 95% credible intervals of posterior distributions (95% CIs). Differences were deemed statistically significant if 95% CIs did not overlap. Graphical ellipses were drawn using the plotSiberObject function in the R package SIBER.
As multiple measurements were made for each individual (i.e. along the baleen), the assumption of complete independence of data points was violated. To overcome this, additional datasets were created by randomly subsampling ten data points from each of the six individuals (n = 60) without replacement 200 times. For each subsampled dataset (n = 200) the Breusch–Godfrey test (Breusch 1978) was used to detect autocorrelation between the residuals (per plate) and removed if significant. For the remaining subsampled datasets (n = 96), the replicated analyses provided similar results to the full dataset (assessed by comparing overlap of 95% Cis of posterior distributions). This suggests that pseudoreplication was not biasing the results (see supplementary Table S4, Figure S7). Therefore, results based on the full dataset are presented in subsequent sections.
Intraspecific variation
Within species, similarity of δ13C and δ15N values from incremental baleen samples for each individual was assessed by comparing overlap of posterior distributions. Posterior distributions were calculated using SIBER (95% CIs), and differences were deemed significant if 95% CIs did not overlap.
Results
Spatial and temporal co-occurrence inferred from whaling catches at SGSSI
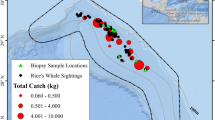
Totals of 16,400 sei whales and 98,843 fin whales were caught within the boundary of the South Georgia and South Sandwich Islands EEZ between 1904 and 1976; location of EEZ is shown in Fig. 1. Fin whales were caught year-round, predominantly between September and May with the highest catches totalling 19,821 (20.1% of all fin whale catches within the SG EEZ) and 28,045 (28.4%) in December and January, respectively (Fig. 2; Table S2). Sei whales were caught from November–May, with the highest catches occurring from February–March (36.4% and 38.7% of all sei whale catches within the SGSSI EEZ, respectively) (Fig. 2; Table S2).
A Location of South Georgia Exclusive Economic Zone (EEZ), South Atlantic, in relation to the Sub-Antarctic Front (dashed line), Polar Front (solid line), and Southern Antarctic Circumpolar Current (dot-dash line). Spatial distribution of fin B and sei C whale catches within the South Georgia Exclusive Economic Zone between 1904 and 1976
A Density distributions of monthly commercial catches of sei whale (blue) and fin whale (orange) within the South Georgia Exclusive Economic Zone between 1904 and 1976. B Cross-correlation functions graphically representing the monthly lag time of sei whale catches compared with fin whale catches. Blue dotted line represents the line of statistical significance at p = 0.05
Intraspecific spatial patterns
Within fin whales, significant differences in the spatial distribution of catches were observed between all time periods (Fig. S4, Table S4). The mean tendency of fin whale catches was at slightly lower latitudes during the early whaling period (< 1929) relative to the mid (1930–1951) to late time periods (> 1952) (Fig. S4). Within sei whales, a significant difference was observed in the spatial distribution of catches between the middle and late time periods (Fig. S4, Table S4). As all sei whale catches occurred within a single hexbin during the early whaling period, correlations between the early time period and later time periods could not be formally tested (Fig S4, Table S4). Sei whales were caught inshore at South Georgia and offshore at lower latitudes within the SGSSI EEZ during the mid (1929–1952) to late (1953–1976) time periods (Fig. S4).
Interspecific spatial patterns
The overall catch distributions significantly differed between the two species within the SGSSI EEZ (Correlation coefficient close to zero, t = 0.008, F = 0.01, df = 1, 188, p = 0.91; Fig. 1). Fin whales were caught across the entire EEZ, with the highest catches recorded close to the island of South Georgia, where the whaling stations were established (Fig. 1). Moderate levels of fin whale catches were also recorded in offshore waters surrounding the island of South Georgia and at a band approximately -59 degrees latitude, towards the lower half of the South Sandwich Islands. Similarly, to fin whales, the highest numbers of sei whale catches were recorded close to the island of South Georgia (Fig. 1). However, in contrast to fin whales, sei whales were caught predominantly at lower latitudes with the percentile of catches much lower at higher latitude regions (e.g. near to the South Sandwich Islands).
Intraspecific temporal patterns
Within species, cross-correlations showed no significant differences in the peak timing of fin whale catches over time, with the highest numbers of fin whales caught in January, consistent across time periods (Table S3, Fig. S3. A–C). Prior to 1929, there were fewer catches of fin whales recorded during the austral winter (June to September) relative to the rest of the year, and no winter catches after 1929 (Table S3, Fig S2). For sei whales, the highest numbers of catches occurred between February and April and peak catch times remained relatively consistent across time periods (Table S3, Fig. S2-S3). A significant difference was observed between the early whaling period (highest in March and April) relative to the middle and late whaling periods (highest in February and March) (Fig. S3.D, E, respectively). No sei whale catches were reported during winter (June to September), and relatively few sei whale catches were made between October and December, consistent across time periods (Table S3, Fig S2–S3).
Interspecific temporal patterns
Throughout the twentieth century, the peak timing of fin whale catches occurred earlier in the season at the SGSSI EEZ compared with sei whales. This was supported by cross-correlation functions which indicated that the peak timing of sei whale catches was at least one month later relative to fin whales (statistically significant lag time of one to two months, Fig. 2, ccf: + 0 − 0.436, + 1− 0.792, + 2 0.804).
Temporal patterns of peak catches were consistent within time periods, with peak fin whale catches occurring earlier in the season compared with sei whales during the early, mid and late whaling periods. (Fig. S2). This was supported by cross-correlation functions with a statistically significant lag time in peak catches between 1 and 3 months depending on time period (Fig. S3). Prior to 1929, the significant lag time was two to three months (Fig. S2, ccf: + 2− 0.666, + 3− 0.599, p < 0.05). In contrast, both the middle (1929–1953) and late catch periods (> 1953) had significant lag times of one to two months (Middle ccf: + 1− 0.783, + 2− 0.846, Fig. S2; Late ccf: + 1− 0.683, + 2− 0.747, Fig. S2, p < 0.05).
Inference of historic resource partitioning using stable isotope analysis
Stable isotope ratios were successfully measured in 111 baleen samples from sei whales (n = 3 plates, with 18, 19 and 20 incremental samples) and fin whales (n = 3 plates, with 15, 19 and 20 incremental samples). Within plates, no correlation was observed between δ13C and C:N suggesting that residual lipids had been removed appropriately (Fig. S1).
Intraspecific variation
Minimum and maximum δ13C values were varied within fin whales (− 20.7 ‰ to − 20.2 ‰; − 23.1 ‰ to − 21.5 ‰; − 21.3 ‰ to − 17.4 ‰) and similar within sei whales (− 19.7 ‰ to − 16.7 ‰; − 21.1 ‰ to − 16.9 ‰; − 19.9.1 ‰ to − 14.3 ‰) (Fig. 3). Minimum and maximum δ15N values were similar within sei whales (7.8 ‰ to − 9.3 ‰; 7.8 ‰ to 10.0 ‰; 7.7 ‰ to 10.2‰) and within fin whales (6.3 ‰ to 7.1 ‰; 6.0 ‰ to 7.6 ‰; 6.5 ‰ to 7.9 ‰)(Fig. 3). Within species, differences in δ15N values between individuals were minimal, with significant overlap of 95% CIs (Fig. 3, Table S5). In contrast, significant intra-specific variation of δ13C was observed between one individual and the other two individuals, consistent for both species (Fig. 3, Table S5). However, posterior distributions from SIBER analysis do not capture the full variation in δ13C and at least some incremental samples along the annual growth cycle overlapped in δ13C within species (Fig. 3, Fig. S5).
A Variation in δ13C values of incremental samples from baleen plates of fin whale (orange) and sei whale (blue). B Variation in δ15N values of incremental samples from baleen plates of fin whale (orange) and sei whale (blue). Youngest to oldest samples are presented from left to right with samples at zero cm close to the gumline. Posterior distributions of stable isotope ratios C δ13C values; and D δ15N values of fin whale Balaenoptera physalus (orange) and sei whale Balaenoptera borealis (blue) baleen collected at South Georgia during the early 1970s extracted from SIBER analysis. Significant differences were assessed by overlap of the 95% credible intervals
Interspecific variation
Sei whale baleen showed significantly higher δ13C and δ15N compared to fin whale baleen (δ13C: W = 263, p < 0.001; δ15N: W = 13, p < 0.001; Fig. 4). Across all incremental sei whale samples, δ15N values ranged from 7.7‰ to 10.2‰ (mean ± SD: 9.1 ± 0.6, n = 57), and δ13C values ranged from − 21.1‰ to − 14.3‰ (mean ± SD: − 17.7 ± 1.6, n = 57). In contrast, across all incremental fin whale samples, δ15N values ranged from 5.9 to 7.9‰ (mean ± SD: 7.03 ± 0.48, n = 54), and δ13C values ranged from -23.1‰ to -17.4‰ (mean ± SD: − 20.7 ± 1.4, n = 54, Fig. 4, Table 1).
A δ15N values and B δ13C values of fin whale (orange circles) and sei whale (blue triangles) baleen collected from ex-whaling sites at South Georgia during the early 1970s. C Bivariate stable isotope ratios (δ13C and δ15N) of incremental baleen samples from fin whale (orange circles) and sei whale (blue triangles) collected at South Georgia during the early 1970s. Bivariate ellipse areas representing 40% (inner contour) and 95% (outer contour) of the data are shown. D Posterior distributions and 95% credible intervals (thin black line) of fin whale (orange) and sei whale (blue) Bayesian standard ellipse areas from SIBER analysis (SEAb)
Sei whale niche area, measured as standard ellipse area, was larger than fin whales (‰2 ± SD: 3.4 ± 0.3, 2.7 ± 0.2, respectively), consistent across 40% and 95% ellipse contours and across maximum likelihood (SEAc) and Bayesian estimates (SEAb) (Fig. 4, Table 2). No overlap in isotopic niches was observed at the core niche level (c.40%) and approximately 9% of overlap was observed when ellipse areas were estimated using 95% of the data (Table 2).
Discussion
Our study is the first to show evidence of interspecific resource partitioning by baleen whales in the Southern Hemisphere during the twentieth-century whaling period using multiple lines of evidence and is the first study using isotopic data to measure resource partitioning between whale species on a summer feeding ground in the South Atlantic. Here, by comparing the spatiotemporal occurrence of fin and sei whales within the SGSSI EEZ during the twentieth century, we showed that sei whales may have occurred at SGSSI later in the season relative to fin whales and that sei whales predominantly utilised the northern part of the EEZ, whilst fin whales were more widely distributed. Historic baleen specimens from the sub-Antarctic during the commercial whaling period are rare; therefore, our sample sizes for isotopic analysis were small (111 incremental samples from 6 individuals). Although confidence in our isotopic results is limited by small sample sizes, the distinct isotopic niches of fin and sei whales when combined with the spatiotemporal differences from catch data provide novel insight into how resource partitioning may have facilitated the coexistence of fin and sei whales at SGSSI during the twentieth-century whaling period.
Interspecific resource partitioning in baleen whales
Here, whaling catch data demonstrated that fin whales were caught year-round at SGSSI, with highest catches during December and January. Comparatively, sei whales were only caught between January and May with highest catches between February and April. This pattern was consistent across time periods (early, middle, late), with the majority of fin whale catches earlier in the season compared with sei whales. Additionally, sei whales were predominantly caught in the northern part of the EEZ, whereas fin whales were caught throughout the region. Both species co-occurred in the late summer and early autumn during the twentieth century within the SGSSI EEZ, which may have increased competition.
Although no study has examined the contemporary spatiotemporal occurrence of fin and sei whales at SGSSI, both species co-occur on feeding grounds in other parts of the globe (Flinn et al. 2002; Frans and Augé 2016; Silva et al. 2019; Buchan et al. 2021; García-Vernet et al. 2021). For example, off the coast of the Falkland Islands in the western South Atlantic, fin and sei whales were both historically observed by local inhabitants predominantly between January and June (Frans and Augé 2016). The Falkland Islands are known feeding grounds for sei whales (Segre et al. 2021); however, limited information is available for fin whales in this region and sightings are rare (GBIF 2021). In the North Pacific, fin and sei whales are observed during summer off the coast of British Columbia; however, sei whales are observed further offshore, likely associated with the abundance and distribution of copepods, whilst fin whales are associated with the availability of euphausiids (Flinn et al. 2002). In the North Atlantic, fin and sei whales co-occur on winter feeding grounds in the Azores (Silva et al. 2019) and similarly to our study, using isotopic niches Silva et al. (2019) showed that present-day populations of fin and sei whales partition resources in the North Atlantic. These examples demonstrate that fin and sei whales co-occur on feeding grounds across the globe.
Despite the seasonal co-occurrence of fin and sei whales at SGSSI, no overlap of the ‘core’ niche (c.40%) and only partial overlap of the entire niche (c.95%) was observed between the fin and sei whales using stable isotope analysis in our study, suggesting that these sympatric species may have partitioned resources on South Atlantic feeding grounds. Resource partitioning has been observed for other sympatric baleen whale species across the globe, including other whale feeding ground sites in the Southern Ocean (Friedlaender et al. 2009; Ryan et al. 2013; Sasaki et al. 2013; Gavrilchuk et al. 2014; Witteveen and Wynne 2016; Herr et al. 2016; Seyboth et al. 2018; Silva et al. 2019; Milmann et al. 2020; Mansouri et al. 2021). For example, Seyboth et al. (2018) found evidence for resource partitioning among fin, humpback, and minke whales on a summer polar feeding ground at the western Antarctic Peninsula (~ 15 degrees further South relative to South Georgia). To date, very few isotope studies have included sei whales (Sasaki et al. 2013; Silva et al. 2019) with no multispecies studies published from the Southern Hemisphere.
Sei whales have been observed foraging at lower latitudes elsewhere in the South Atlantic, including coastal Africa (Best 1967; Best and Gambell 1968; Best and Lockyer 2002). In our study, all three sei whales had much higher δ13C across the majority of their incremental samples compared with fin whales, consistent with foraging at lower latitudes across the year (as lower δ13C values occur nearer the poles, see Cherel and Hobson 2007; Magozzi et al. 2017; Espinasse et al. 2019). Moreover, the distribution of whaling catches circumpolar suggest sei whales foraged across a lower latitudinal range relative to fin whales in the Southern Hemisphere, with fin whales reported at both sub-polar and polar latitudes, and adult sei whales only occasionally reported at polar latitudes (Mizroch et al. 1984; Mizroch and Rice 1984; Horwood 1987; Kasamatsu 1996, 2000). This pattern was reflected by the catch data presented in our study with sei whales occurring predominantly within the northern part of the SGSSI EEZ, whilst fin whales occurred throughout; therefore, the higher δ13C across the majority of sei whale samples may have been due to relatively lower latitude foraging by sei whales in sub-polar habitats.
It is important to note that our results were based on 111 incremental baleen samples from only six individuals (3 fin, 3 sei), and therefore, further samples would be required to interpret the observed patterns in a population context. Nevertheless, historic whale specimens from the Southern Ocean are rare and dietary information for fin and sei whales is currently extremely limited at SGSSI; therefore, our study provides novel information on historical foraging strategies in these two species for an understudied part of the Southern Ocean, known to represent vital whale feeding habitat (Atkinson et al. 2001; Jackson et al. 2020). Further isotopic information on fin and sei whales in the Southern Ocean will help to determine whether these two sympatric species still co-occur and partition resources at SGSSI and how they may partition resources with other Balaenoptera whale species.
Foraging ecology of krill predators at South Georgia
South Georgia is well known for its iconic marine megafauna associated with high levels of primary productivity and dense aggregations of zooplankton. Many penguin, fish, seal, and seabird colonies inhabit the region with somewhat overlapping diets, many of which forage on Antarctic krill (Bearhop et al. 2006; Phillips et al. 2011; Waluda et al. 2017; Horswill et al. 2018; Jones et al. 2020; Mills et al. 2020, 2021; Hollyman et al. 2021). Direct information on fin and sei whale diet is limited to studies that reported stomach contents during the commercial whaling period. These studies suggest fin whales fed predominantly on euphausiid krill across the Southern Ocean (Mackintosh and Wheeler 1929; Mackintosh 1942; Nemoto and Nasu 1958; Nemoto 1959; Kawamura 1980), whilst sei whales had a broader diet, including copepods, amphipods, and decapods (Nemoto 1959; Nemoto 1962; Klumov 1963; Brown 1968; Nemoto 1970; Kawamura 1974; Budylenko 1978). At South Georgia, fin whales and sei whales foraged on euphausiids (krill) with some evidence of sei whales supplementing the diet with amphipods at low and high latitudes during the twentieth century (Matthews 1938; Brown 1968).
Predators that forage on similar food items at the same time of year are likely to display similar stable isotope ratios (δ13C, δ15N, see Lepoint and Das 2011). In our study, stable isotope ratios of fin whale baleen (means ± SDs: δ13C, − 20.7 ± 1.4 ‰, δ15N, 7.0 ± 0.5 ‰) were similar to various present-day krill predators at South Georgia, including southern right whales (− 21.0 ± 0.4 ‰, 8.2 ± 0.8 ‰), male Antarctic fur seals (− 21.7 ± 1.2 ‰, δ15N, 9.0 ± 1.0 ‰), gentoo penguins (− 18.9 ± 0.3 ‰, 8.6 ± 0.3 ‰) and macaroni penguins (− 20.0 ± 0.6 ‰, 8.9 ± 0.4 ‰), whilst sei whale values were similar albeit slightly higher (δ13C, − 17.7 ± 1.6 ‰, δ15N, 9.1 ± 0.6 ‰) (Table 3). Although historic and present-day isotopic data are not directly comparable due to temporal and spatial changes in isotopic baselines (Keeling 1979; Misarti et al. 2009, 2017; Baker et al. 2010; Eide et al. 2017), the similarity in isotopes between twenty-first century krill predators and twentieth-century whales (Table 3), alongside the historic diet data (Matthews 1938; Brown 1968), provide some indication that fin and sei whales may have consumed krill at South Georgia during the twentieth century. Additionally, although the lowest paired values of sei whale δ13C and δ15C values are similar to krill predators at South Georgia (Table 3), many of the higher sei whale δ13C values (total range − 21.1‰ to − 14.3‰) fall outside the observed range measured for other predators. This information, combined with the short occupancy time of sei whales at South Georgia inferred from catch data, could suggest that sei whales were feeding on alternative prey sources at lower latitudes when not on the South Georgia feeding grounds. This hypothesis is supported by observations of sei whales foraging on low-trophic zooplankton at lower latitudes during winter (Best and Gambell 1968; Horwood 1987; Prieto et al. 2012; Silva et al. 2019). Further research on sei whale diet throughout their foraging range is required to understand whether sei whales are supplementing higher trophic prey at SGSSI, or whilst at lower latitudes (prior to arrival on the feeding grounds).
Intra-individual variation and evidence of winter foraging in fin and sei whales
South Georgia and the South Sandwich Islands are thought to represent a long-term (historical and contemporary) feeding ground for both species; however, there is limited information on the precise migratory routes and wintering locations for South Atlantic fin and sei whales populations that forage at SGSSI (Mackintosh 1942; Mikhalev 2020). There is some evidence to suggest that the Brazilian coast (Andriolo et al. 2010; Weir et al. 2020) and southwest coast of Africa represent wintering grounds for sei whales (Best 1967; Best and Gambell 1968; Best and Lockyer 2002; Best and Folkens 2007); and our stable isotope patterns also support the hypothesis that sei whales at South Georgia may have been feeding at low latitudes in winter (demonstrated by the higher δ13C values, Fig. 3). Fin whales have also been observed off the South African coast during the austral winter (Best 1967; Best and Folkens 2007; Shabangu et al. 2019). Historically, fin whales have been thought to fast during winter on migration (Mackintosh and Wheeler 1929; Best and Folkens 2007). In our study, patterns of δ13C values from one out of three fin whale plates and all three sei whale plates are consistent with winter foraging at lower latitudes, demonstrated by δ13C values above − 20‰, followed by a dramatic reduction in δ13C between 5 and 10 cm associated with a temporary shift towards polar feeding (Fig. 3). Indeed, acoustic detections suggest fin whales may seasonally forage on temperate krill species such as Nematoscelis megalops at lower latitudes in the productive Benguela ecosystem (Shabangu et al. 2019). The other two fin whale plates in our study show consistent δ13C values throughout the year (Fig. 3), suggesting they either maintain polar foraging year-round, or fast when migrating to lower latitudes. Similar patterns of intra-specific variation have been previously observed in the Southern Hemisphere for humpback whales (Eisenmann et al. 2016) and southern right whales (Rowntree et al. 2008), where some individuals fast and others forage at low latitudes. Further research is needed to establish migratory connectivity between SGSSI and lower latitude wintering grounds for fin and sei whales, and to better understand the occurrence of foraging on wintering grounds for both species.
Possible biases of whaling catch data
In this study, we observed many catches of fin and sei whales nearshore to the island of South Georgia during the early whaling period (< 1929). It is possible that these nearshore catches represent the plentiful whale numbers that occurred nearshore during this time, or, they may also reflect whalers recording catches at the time of landing, rather than at sea during incidence of capture (Tønnessen and Johnsen 1982). As whaling was predominantly close to the shoreline during the early whaling period (until steam-powered catcher vessel improved in the 1920s) and reporting generally improved throughout the twentieth-century (Tønnessen and Johnsen 1982), it is likely to be a combination of these scenarios. Despite these known biases, we did not exclude this early whaling period from the results for several reasons. First, the initial whaling period at South Georgia was close to shore and is documented as plentiful with approximately 100,000 baleen whales caught close to South Georgia prior to 1929 including large numbers of fin and sei whales (Allison 2016). Second, prior to the 1920s, whalers were not using steam-powered capture vessels, and therefore, whaling further offshore in sub-Antarctic waters was unlikely during the initial period. Third, although some variation was observed in spatial distribution of fin and sei whales across time, fin whales were caught throughout the EEZ even during the early period, and sei whales were consistently caught at higher densities in the northern range of the EEZ (Fig. S4). Moreover, whaler preference for specific species shifted throughout the commercial whaling period in the following order: (1st) humpback, (2nd) blue, (3rd) fin, (4th) sei, (5th) minke. Fin whales were the main target between 1937 and 1965, whereas sei whales were the dominant target between 1965 and 1975 (once fin whale abundance had significantly diminished, Tønnessen and Johnsen (1982), pp.164–165). Fin whales were caught throughout the EEZ despite being targeted prior to sei whales, suggesting whaling was already occurring across the entire EEZ before the majority of sei whaling occurred; therefore, we assume here that later catches were not strongly biased by whaler presence and that the whaling catches reported across the EEZ in summary provide a good indication of feeding ground distribution of sei and fin whales at SGSSI during the twentieth century.
Conclusion
Fin and sei whales co-occurred at South Georgia and the South Sandwich Islands (SGSSI) during the twentieth-century whaling period predominantly during late summer and early autumn. Here, we have provided the first isotopic information for fin and sei whales in this region and by combining isotopic evidence with spatiotemporal information from whaling catch data we show evidence of potential resource partitioning during the commercial whaling period, an era when whale numbers were much higher (Rocha et al. 2015) and competition for resources potentially heightened. Temporal and spatial variation in whaling catches suggests spatiotemporal differences in habitat use of fin and sei whales at SGSSI with the highest numbers of sei whales occurring later in the season and utilising the northern part of the exclusive economic zone compared with fin whales, with both species found nearshore and offshore. Although sample sizes were small, stable isotope analysis of baleen plates suggest limited interspecific overlap in isotopic niches indicative of resource partitioning. Sequential δ13C values of baleen plates provide evidence of individuals from both species foraging at lower latitudes prior to arrival at SGSSI during the twentieth-century whaling period. Further dietary and isotopic information (e.g. from baleen of deceased strandings or live skin biopsies) in present-day fin and sei whale populations are needed to understand the various roles these whales play in the SGSSI ecosystem, including any overlap that may occur with neighbouring marine predators, and commercial fisheries.
Data availability
The data sets generated during the current study are available from the corresponding author on reasonable request.
References
Allison C (2016) IWC Individual Catch Database Version 6.1. Available on request from statistics@iwc.int; International Whaling Commission: Cambridge, UK.
Andriolo A, da Rocha JM, Zerbini AN, Simoes-Lopes PC, Moreno IB, Lucena A, Danilewicz D, Bassoi M (2010) Distribution and relative abundance of large whales in a former whaling ground off eastern South America. Zoologia 27:741–750. https://doi.org/10.1590/S1984-46702010000500011
Atkinson A, Whitehouse MJ, Priddle J, Cripps GC, Ward P, Brandon MA (2001) South Georgia, Antarctica: a productive, cold water, pelagic ecosystem. Mar Ecol Prog Ser 216:279–308. https://doi.org/10.3354/meps216279
Baines M, Kelly N, Reichelt M, Lacey C, Pinder S, Fielding S, Murphy E, Trathan P, Biuw M, Lindstrøm U, Krafft BA, Jackson JA (2021) Population abundance of recovering humpback whales Megaptera novaeangliae and other baleen whales in the Scotia Arc, South Atlantic. Mar Ecol Prog Ser 676:77–94. https://doi.org/10.3354/meps13849
Baker DM, Webster KL, Kim K (2010) Caribbean octocorals record changing carbon and nitrogen sources from 1862 to 2005. Glob Chang Biol 16:2701–2710. https://doi.org/10.1111/j.1365-2486.2010.02167.x
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164. https://doi.org/10.3354/meps311157
Bentaleb I, Martin C, Vrac M, Mate B, Mayzaud P, Siret D, De Stephanis R, Guinet C (2011) Foraging ecology of Mediterranean fin whales in a changing environment elucidated by satellite tracking and baleen plate stable isotopes. Mar Ecol Prog Ser 438:285–302
Best PB (1967) Distribution and feeding habits of baleen whales off the cape province republic of South Africa, department of commerce and industries. Invest Rep Div Sea Fish South Af 57:1–44
Best PB, Folkens PA (2007) Whales and dolphins of the southern African subregion. Cambridge University Press, Cape Town
Best PB, Gambell R (1968) The abundance of sei whales off South Africa. Norsk Hvalfangsttid 57:168–174
Best PB, Lockyer CH (2002) Reproduction, growth and migrations of sei whales Balaenoptera borealis off the west coast of South Africa. Afr J Mar Sci 24:111–133
Best PB, Schell DM (1996) Stable isotopes in southern right whale (Eubalaena australis) baleen as indicators of seasonal movements, feeding and growth. Mar Biol 124:483–494. https://doi.org/10.1007/BF00351030
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable. Isotop Troph Ecol. https://doi.org/10.1146/annurev-ecolsys-102209-144726
Borrell A, Abad-Oliva N, Gómez-Campos E, Giménez J, Aguilar A (2012) Discrimination of stable isotopes in fin whale tissues and application to diet assessment in cetaceans. Rapid Commun Mass Spectrom 26:1596–1602. https://doi.org/10.1002/rcm.6267
Bowen WD, Iverson SJ (2012) Methods of estimating marine mammal diets: a review of validation experiments and sources of bias and uncertainty. Mar Mamm Sci. https://doi.org/10.1111/j.1748-7692.2012.00604.x
Breusch TS (1978) Testing for autocorrelation in dynamic linear models. Aust Econ Pap 17:334–355. https://doi.org/10.1111/j.1467-8454.1978.tb00635.x
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7:434–455
Brown SG (1968) Feeding of sei whales at South Georgia. Norsk Hvalfangsttidende 57:118–125
Buchan SJ, Vásquez P, Olavarría C, Castro LR (2021) Prey items of baleen whale species off the coast of Chile from fecal plume analysis. Mar Mamm Sci 37:1116–1127. https://doi.org/10.1111/mms.12782
Budylenko GA (1978) On sei whale feeding in the southern ocean. Rep Int Whal Comm 28:379–385
Cade DE, Seakamela SM, Findlay KP, Fukunaga J, Kahane-Rapport SR, Warren JD, Calambokidis J, Fahlbusch JA, Friedlaender AS, Hazen EL, Kotze D, McCue S, Meÿer M, Oestreich WK, Oudejans MG, Wilke C, Goldbogen JA (2021a) Predator-scale spatial analysis of intra-patch prey distribution reveals the energetic drivers of rorqual whale super-group formation. Funct Ecol 35:894–908. https://doi.org/10.1111/1365-2435.13763
Cade DE, Fahlbusch JA, Oestreich WK, Ryan J (2021b) Social exploitation of extensive, ephemeral, environmentally controlled prey patches by supergroups of rorqual whales. Anim Behav 182:251–266
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287. https://doi.org/10.3354/meps329281
Cherel Y, Phillips RA, Hobson KA, McGill R (2006) Stable isotope evidence of diverse species-specific and individual wintering strategies in seabirds. Biol Lett 2:301–303. https://doi.org/10.1098/rsbl.2006.0445
Cherel Y, Koubbi P, Giraldo C, Penot F, Tavernier E, Moteki M, Ozouf-Costaz C, Causse R, Chartier A, Hosie G (2011) Isotopic niches of fishes in coastal, neritic and oceanic waters off Adélie land, Antarctica. Polar Sci 5:286–297. https://doi.org/10.1016/j.polar.2010.12.004
Clapham P, Good C, Quinn S, Reeves RR, Scarff JE, Brownell RL (2004) Distribution of North Pacific right whales (Eubalaena japonica) as shown by 19th and 20th century whaling catch and sighting records. J Cetacean Res Manage 6:1–6
Clavero M (2014) Shifting baselines and the conservation of non-native species. Conserv Biol 28:1434–1436. https://doi.org/10.1111/cobi.12266
Clifford P, Richardson S, Hémon D (1989) Assessing the significance of the correlation between two spatial processes. Biometrics 45:123–134
Dabney J, Knapp M, Glocke I, Gansauge M-T, Weihmann A, Nickel B, Valdiosera C, García N, Pääbo S, Arsuaga J-L, Meyer M (2013) Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc Natl Acad Sci U S A 110:15758–15763. https://doi.org/10.1073/pnas.1314445110
Derrick TR, Thomas JM (2004) Chapter 7. Time-series analysis: the cross-correlation function. In: Stergiou N (ed) Innovative analyses of human movement. Human Kinetics Publishers, Champaign, IL, pp 189–205. https://dr.lib.iastate.edu/handle/20.500.12876/52528
Doughty CE, Roman J, Faurby S, Wolf A, Haque A, Bakker ES, Malhi Y, Dunning JB Jr, Svenning J-C (2016) Global nutrient transport in a world of giants. Proc Natl Acad Sci USA 113:868–873. https://doi.org/10.1073/pnas.1502549112
Drago M, Cardona L, Franco-Trecu V, Crespo EA, Vales DG, Borella F, Zenteno L, Gonzáles EM, Inchausti P (2017) Isotopic niche partitioning between two apex predators over time. J Anim Ecol 86:766–780. https://doi.org/10.1111/1365-2656.12666
Durante CA, Crespo EA, Loizaga R (2021) Isotopic niche partitioning between two small cetacean species. Mar Ecol Prog Ser 659:247–259. https://doi.org/10.3354/meps13575
Dutilleul P, Clifford P, Richardson S, Hemon D (1993) Modifying the t test for assessing the correlation between two spatial processes. Biometrics 49:305–314. https://doi.org/10.2307/2532625
Eide M, Olsen A, Ninnemann US, Eldevik T (2017) A global estimate of the full oceanic 13 C Suess effect since the preindustrial. Global Biogeochem Cycles 31:492–514. https://doi.org/10.1002/2016gb005472
Eisenmann P, Fry B, Holyoake C, Coughran D, Nicol S, Bengtson Nash S (2016) Isotopic evidence of a wide spectrum of feeding strategies in southern hemisphere humpback whale baleen records. PLoS ONE. https://doi.org/10.1371/journal.pone.0156698
Espinasse B, Pakhomov EA, Hunt BPV, Bury SJ (2019) Latitudinal gradient consistency in carbon and nitrogen stable isotopes of particulate organic matter in the Southern Ocean. Mar Ecol Prog Ser 631:19–30. https://doi.org/10.3354/meps13137
Fanelli E, Badalamenti F, D’Anna G, Pipitone C, Riginella E, Azzurro E (2011) Food partitioning and diet temporal variation in two coexisting sparids, Pagellus erythrinus and Pagellus acarne. J Fish Biol 78:869–900. https://doi.org/10.1111/j.1095-8649.2011.02915.x
Findlay KP, Mduduzi Seakamela S, Meÿer MA, Kirkman SP, Barendse J, Cade DE, Hurwitz D, Kennedy AS, Kotze PGH, McCue SA, Thornton M, Alejandra Vargas-Fonseca O, Wilke CG (2017) Humpback whale “super-groups”: A novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. PLoS ONE. https://doi.org/10.1371/journal.pone.0172002
Flinn RD, Trites AW, Gregr EJ, Perry RI (2002) Diets of fin, sei, and sperm whales in British Columbia: an analysis of commercial whaling records, 1963–1967. Mar Mamm Sci 18:663–679
Fossette S, Abrahms B, Hazen EL, Bograd SJ, Zilliacus KM, Calambokidis J, Burrows JA, Goldbogen JA, Harvey JT, Marinovic B, Tershy B, Croll DA (2017) Resource partitioning facilitates coexistence in sympatric cetaceans in the California Current. Ecol Evol 7:9085–9097. https://doi.org/10.1002/ece3.3409
Francois R, Altabet MA, Goericke R, McCorkle DC, Brunet C, Poisson A (1993) Changes in the δ13C of surface water particulate organic matter across the subtropical convergence in the SW Indian Ocean. Global Biogeochem Cycles 7:627–644. https://doi.org/10.1029/93gb01277
Frans VF, Augé AA (2016) Use of local ecological knowledge to investigate endangered baleen whale recovery in the Falkland Islands. Biol Conserv 202:127–137. https://doi.org/10.1016/j.biocon.2016.08.017
Friedlaender AS, Lawson GL, Halpin PN (2009) Evidence of resource partitioning between humpback and minke whales around the western Antarctic Peninsula. Mar Mamm Sci 25:402–415. https://doi.org/10.1111/j.1748-7692.2008.00263.x
Friedlaender AS, Goldbogen JA, Hazen EL, Calambokidis J, Southall BL (2015) Feeding performance by sympatric blue and fin whales exploiting a common prey resource. Mar Mamm Sci 31:345–354. https://doi.org/10.1111/mms.12134
Friedlaender AS, Bowers MT, Cade D (2020) The advantages of diving deep: fin whales quadruple their energy intake when targeting deep krill patches. Func Ecol 34:497–506
Friedlaender AS, Joyce T, Johnston DW, Read AJ, Nowacek DP, Goldbogen JA, Gales N, Durban JW (2021) Sympatry and resource partitioning between the largest krill consumers around the Antarctic Peninsula. Mar Ecol Prog Ser 669:1–16. https://doi.org/10.3354/meps13771
Fry B, Sherr EB (1989) δ13C Measurements as Indicators of Carbon Flow in Marine and Freshwater Ecosystems. Stable Isotopes in Ecological Research. Springer, New York, pp 196–229
García-Vernet R, Borrell A, Víkingsson G, Halldórsson SD, Aguilar A (2021) Ecological niche partitioning between baleen whales inhabiting Icelandic waters. Prog Oceanogr. https://doi.org/10.1016/j.pocean.2021.102690
Garneau DE, Post E, Boudreau T, Keech M, Valkenburg P (2007) Spatio-temporal patterns of predation among three sympatric predators in a single-prey system. Wbio 13:186–194. https://doi.org/10.2981/0909-6396(2007)13[186:SPOPAT]2.0.CO;2
Gavrilchuk K, Lesage V, Ramp C, Sears R, Bérubé M, Bearhop S, Beauplet G (2014) Trophic niche partitioning among sympatric baleen whale species following the collapse of groundfish stocks in the Northwest Atlantic. Mar Ecol Prog Ser 497:285–301
GBIF Secretariat (2021) GBIF backbone taxonomy. Checklist dataset. https://doi.org/10.15468/39omei. Accessed 28 Oct 2020
Gelman A, Rubin DB (1992) Inference from Iterative Simulation Using Multiple Sequences. SSO Schweiz Monatsschr Zahnheilkd 7:457–472. https://doi.org/10.1214/ss/1177011136
Gerrish GA, Morin JG (2016) Living in sympatry via differentiation in time, space and display characters of courtship behaviors of bioluminescent marine ostracods. Mar Biol 163:190. https://doi.org/10.1007/s00227-016-2960-5
Goldbogen JA, Cade DE, Calambokidis J, Friedlaender AS, Potvin J, Segre PS, Werth AJ (2017) How baleen whales feed: the biomechanics of engulfment and filtration. Ann Rev Mar Sci 9:367–386. https://doi.org/10.1146/annurev-marine-122414-033905
Gordon JE, Haynes VM, Hubbard A (2008) Recent glacier changes and climate trends on South Georgia. Glob Planet Change 60:72–84. https://doi.org/10.1016/j.gloplacha.2006.07.037
Grinnell J (1924) Geography and evolution. Ecology 5:225–229. https://doi.org/10.2307/1929447
Gulka J, Ronconi RA, Davoren GK (2019) Spatial segregation contrasting dietary overlap: niche partitioning of two sympatric alcids during shifting resource availability. Mar Biol. https://doi.org/10.1007/s00227-019-3553-x
Hammerschlag N, Luo J, Irschick DJ, Ault JS (2012) A comparison of spatial and movement patterns between sympatric predators: bull sharks (Carcharhinus leucas) and Atlantic tarpon (Megalops atlanticus). PLoS ONE. https://doi.org/10.1371/journal.pone.0045958
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297. https://doi.org/10.1126/science.131.3409.1292
Headland R (1992) The Island of South Georgia. Cambridge University Press, Cambridge, UK, CUP Archive
Healy K, Kelly SB, Guillerme T, Inger R, Bearhop S, Jackson AL (2017) Predicting trophic discrimination factor using Bayesian inference and phylogenetic, ecological and physiological data. DEsIR: Discrimination Estimation in R (No. e1950v3). PeerJ Preprints
Herr H, Viquerat S, Siegel V, Kock K-H, Dorschel B, Huneke WGC, Bracher A, Schröder M, Gutt J (2016) Horizontal niche partitioning of humpback and fin whales around the West Antarctic Peninsula: evidence from a concurrent whale and krill survey. Polar Biol 39:799–818. https://doi.org/10.1007/s00300-016-1927-9
Hill SL, Phillips T, Atkinson A (2013) Potential climate change effects on the habitat of antarctic krill in the weddell quadrant of the southern ocean. PLoS ONE. https://doi.org/10.1371/journal.pone.0072246
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326. https://doi.org/10.1007/s004420050865
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798. https://doi.org/10.2307/5256
Hoefs J (2018) Stable Isotope Geochemistry. Springer, Berlin, Germany
Hollyman PR, Hill SL, Laptikhovsky VV, Belchier M, Gregory S, Clement A, Collins MA (2021) A long road to recovery: dynamics and ecology of the marbled rockcod (Notothenia rossii, family: Nototheniidae) at South Georgia, 50 years after overexploitation. ICES J Mar Sci 78:2745–2756
Hoos LA, Buckel JA, Boyd JB, Loeffler MS, Lee LM (2019) Fisheries management in the face of uncertainty: Designing time-area closures that are effective under multiple spatial patterns of fishing effort displacement in an estuarine gill net fishery. PLoS ONE. https://doi.org/10.1371/journal.pone.0211103
Horswill C, Jackson JA, Medeiros R, Nowell RW (2018) Minimising the limitations of using dietary analysis to assess foodweb changes by combining multiple techniques. Ecol Indic 94:218–225
Horwood J (1987) The Sei Whale: Population Biology. Academic Press.
Hutchings L, van der Lingen CD, Shannon LJ, Crawford RJM, Verheye HMS, Bartholomae CH, van der Plas AK, Louw D, Kreiner A, Ostrowski M, Fidel Q, Barlow RG, Lamont T, Coetzee J, Shillington F, Veitch J, Currie JC, Monteiro PMS (2009) The benguela current: an ecosystem of four components. Prog Oceanogr 83:15–32. https://doi.org/10.1016/j.pocean.2009.07.046
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602
Jackson JA, Kennedy A, Moore M, Andriolo A, Bamford CCG, Calderan S, Cheeseman T, Gittins G, Groch K, Kelly N, Leaper R, Leslie MS, Lurcock S, Miller BS, Richardson J, Rowntree V, Smith P, Stepien E, Stowasser G, Trathan P, Vermeulen E, Zerbini AN, Carroll EL (2020) Have whales returned to a historical hotspot of industrial whaling? The pattern of southern right whale Eubalaena australis recovery at South Georgia. Endanger Species Res 43:323–339. https://doi.org/10.3354/esr01072
Jones KA, Ratcliffe N, Votier SC, Newton J, Forcada J, Dickens J, Stowasser G, Staniland IJ (2020) Intra-specific Niche Partitioning in Antarctic Fur Seals. Arctocephalus Gazella Sci Rep 10:3238. https://doi.org/10.1038/s41598-020-59992-3
Kahane-Rapport SR, Savoca MS, Cade DE, Segre PS, Bierlich KC, Calambokidis J, Dale J, Fahlbusch JA, Friedlaender AS, Johnston DW, Werth AJ, Goldbogen JA (2020) Lunge filter feeding biomechanics constrain rorqual foraging ecology across scale. J Exp Biol. https://doi.org/10.1242/jeb.224196
Kasamatsu F (2000) Species diversity of the whale community in the Antarctic. Mar Ecol Prog Ser 200:297–301. https://doi.org/10.3354/meps200297
Kasamatsu, (1996) Current occurrence of baleen whales in the Antarctic waters. Rep Int Whal Commn 46:293–304
Kawamura A (1980) A review of food of balaenopterid whales. Sci Rep Whales Res Inst 32:155–197
Kawamura, (1974) Food and feeding ecology in the southern sei whale. Sci Rep Whale Res Inst 26:25–144
Keeling CD (1979) The Suess effect: 13Carbon-14Carbon interrelations. Environ Int 2:229–300. https://doi.org/10.1016/0160-4120(79)90005-9
Kelt DA, Van Vuren D (1999) Energetic constraints and the relationship between body size and home range area in mammals. Ecology 80:337–340. https://doi.org/10.1890/0012-9658(1999)080[0337:ecatrb]2.0.co;2
Kennedy AS, Carroll E, Baker CS, Bassoi M, Buss D, Collins MA, Calderan S, Ensor P, Fielding S, Leaper R, Macdonald D, Olsen PA, Cheeseman T, Groch KR, Hall A, Kelly N, Miller BS, M. Moore, Rowntree V, Stowasser G, Trathan P, Valenzuela LO, E. Vermeulen, Zerbini A, Jackson JA (2020) Whales return to the epicentre of whaling? Preliminary results from the 2020 cetacean survey at South Georgia (Islas Georgias del Sur) Technical Report. IWC 2020. Annex H.
Klumov KS (1963) Feeding and halminth fauna of whalebone whales (Mystacoceti). Trudy Inst Okeanol 71:94–194
Kopp D, Lefebvre S, Cachera M, Villanueva MC, Ernande B (2015) Reorganization of a marine trophic network along an inshore–offshore gradient due to stronger pelagic–benthic coupling in coastal areas. Prog Oceanogr 130:157–171. https://doi.org/10.1016/j.pocean.2014.11.001
Lea JSE, Humphries NE, Bortoluzzi J, Daly R, von Brandis RG, Patel E, Patel E, Clarke CR, Sims DW (2020) At the turn of the tide: space use and habitat partitioning in two sympatric shark species is driven by tidal phase. Front Mar Sci 7:624. https://doi.org/10.3389/fmars.2020.00624
Leal GR, Bugoni L (2021) Individual variability in habitat, migration routes and niche used by Trindade petrels. Pterodroma Arminjoniana Mar Biol 168:134. https://doi.org/10.1007/s00227-021-03938-4
Leibold MA (1995) The niche concept revisited: Mechanistic models and community context. Ecology 76:1371–1382. https://doi.org/10.2307/1938141
Lepoint, G. and Das, K., 2011, April. You are what you eat, plus a few per mille": apport des isotopes stables en écologie marine PARTIM 1: Introduction et Application générale. In Cours Conférence Collège Belgique
Levin SA (2000) Multiple scales and the maintenance of biodiversity. Ecosystems 3:498–506. https://doi.org/10.1007/s100210000044
Linnebjerg JF, Fort J, Guilford T, Reuleaux A, Mosbech A, Frederiksen M (2013) Sympatric breeding auks shift between dietary and spatial resource partitioning across the annual cycle. PLoS ONE. https://doi.org/10.1371/journal.pone.0072987
Lopez-Lopez L, Preciado I, Velasco F, Olaso I, Gutiérrez-Zabala JL (2011) Resource partitioning amongst five coexisting species of gurnards (Scorpaeniforme: Triglidae): Role of trophic and habitat segregation. J Sea Res 66:58–68. https://doi.org/10.1016/j.seares.2011.04.012
MacFarland TW, Yates JM (2016) Mann-Whitney U Test. In: MacFarland TW, Yates JM (eds) Introduction to Nonparametric Statistics for the Biological Sciences Using R. Springer International Publishing, Cham, pp 103–132
Mackintosh AN (1942) The southern stocks of whalebone whales. Discovery Rep 22:197–300
Mackintosh NA (1946) The natural history of whalebone whales. Biol Rev Camb Philos Soc 21:60–74. https://doi.org/10.1111/j.1469-185x.1946.tb00453.x
Mackintosh A, Wheeler JFG (1929) Southern blue and fin whales. Discovery Rep 1:257–540
Magozzi S, Yool A, Vander Zanden HB, Wunder MB, Trueman CN (2017) Using ocean models to predict spatial and temporal variation in marine carbon isotopes. Ecosphere. https://doi.org/10.1002/ecs2.1763
Mansouri F, Winfield ZC, Crain DD, Morris B, Charapata P, Sabin R, Potter CW, Hering AS, Fulton J, Trumble SJ, Usenko S (2021) Evidence of multi-decadal behavior and ecosystem-level changes revealed by reconstructed lifetime stable isotope profiles of baleen whale earplugs. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.143985
Matthews LH (1938) The sei whale. University Press, Balaenoptera borealis
McCarthy A, De Robertis A, Kotwicki S, Hough K, Wade P, Wilson C (2021) Differing prey associations and habitat use suggest niche partitioning by fin and humpback whales off Kodiak Island. Mar Ecol Prog Ser 662:181–197. https://doi.org/10.3354/meps13596
McClenachan L, Ferretti F, Baum JK (2012) From archives to conservation: why historical data are needed to set baselines for marine animals and ecosystems. Conserv Lett 5:349–359. https://doi.org/10.1111/j.1755-263x.2012.00253.x
Michel LN, Bell JB, Dubois SF, Le Pans M, Lepoint G, Olu K, Reid WDK, Sarrazin J, Schaal G, Hayden B (2020) DeepIso: a global open database of stable isotope ratios and elemental contents for deep-sea ecosystems. ORBI. https://doi.org/10.17882/76595
Mikhalev Y (2020) Whales of the Southern Ocean: biology, whaling and perspectives of population recovery, vol 5. Springer, Cham
Mills WF, McGill RAR, Cherel Y, Votier SC, Phillips RA (2020) Stable isotopes demonstrate intraspecific variation in habitat use and trophic level of non-breeding albatrosses. Ibis. https://doi.org/10.1111/ibi.12874
Mills WF, Morley TI, Votier SC, Phillips RA (2021) Long-term inter- and intraspecific dietary variation in sibling seabird species. Mar Biol 168:31. https://doi.org/10.1007/s00227-021-03839-6
Milmann L, de Oliveira LR, Danilevicz IM, Di Beneditto APM, Botta S, Siciliano S, Baumgarten J (2020) Stable isotopes analysis on baleen whales (Suborder: Mysticeti): a review until 2017. Boletim Do Laboratorio De Hidrobiologia. https://doi.org/10.18764/1981-6421e2020.10
Misarti N, Finney B, Maschner H, Wooller MJ (2009) Changes in northeast Pacific marine ecosystems over the last 4500 years: evidence from stable isotope analysis of bone collagen from archeological middens. Holocene 19:1139–1151. https://doi.org/10.1177/0959683609345075
Misarti N, Gier E, Finney B, Barnes K, McCarthy M (2017) Compound-specific amino acid δ15 N values in archaeological shell: Assessing diagenetic integrity and potential for isotopic baseline reconstruction. Rapid Commun Mass Spectrom 31:1881–1891. https://doi.org/10.1002/rcm.7963
Mizroch SA, Rice DW (1984) The fin whale, balaenoptera. Mar Fish Rev 46:20–24
Mizroch SA, Rice DW, Breiwick JM (1984) The sei whale, Balaenoptera borealis. Mar Fish Rev 46:25–29
Morera-Pujol V, Ramos R, Pérez-Méndez N, Cerdà-Cuéllar M, González-Solís J (2018) Multi-isotopic assessments of spatio-temporal diet variability: the case of two sympatric gulls in the western Mediterranean. Mar Ecol Prog Ser 606:201–214. https://doi.org/10.3354/meps12763
Navarro J, Cardador L, Brown R, Phillips RA (2015) Spatial distribution and ecological niches of non-breeding planktivorous petrels. Sci Rep 5:12164. https://doi.org/10.1038/srep12164
Nemoto T (1959) Food of baleen whales with reference to whale movements. Sci Rep Whales Res Inst Tokyo 14:149–291
Nemoto T (1962) Food of baleen whales collected in recent Japanese Antarctic whaling expeditions. Sci Rep Whales Res Inst 16:89–103
Nemoto T, Nasu K (1958) Thysanoessa macrura as a food of baleen whales in the Antarctic. Sci Rep Whales Res Inst 13:193–199
Nemoto T (1970) Feeding pattern of baleen whales. Marine Food Chains University of California Press, Berkeley 241–252
Newsome SD, Martinez del Rio C (2007) A niche for isotopic ecology. Front in Ecol and Env 5:4290436. https://doi.org/10.1890/060150.1
Newsome SD, Clementz MT, Koch PL (2010a) Using stable isotope biogeochemistry to study marine mammal ecology. Mar Mamm Sci 26:509–572. https://doi.org/10.1111/j.1748-7692.2009.00354.x
Newsome SD, Bentall GB, Tinker MT, Oftedal OT, Ralls K, Estes JA, Fogel ML (2010b) Variation in δ13C and δ15N diet–vibrissae trophic discrimination factors in a wild population of California sea otters. Ecol Appl 20:1744–1752. https://doi.org/10.1890/09-1502.1
O’Connell TC (2017) ‘Trophic’ and ‘source’ amino acids in trophic estimation: a likely metabolic explanation. Oecologia 184:317–326
O’Connell TC, Hedges RE (1999) Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol 108:409–425. https://doi.org/10.1002/(SICI)1096-8644(199904)108:4%3c409::AID-AJPA3%3e3.0.CO;2-E
O’Connell TC, Hedges REM, Healey MA, Simpson AHRW (2001) Isotopic comparison of hair, nail and bone: modern analyses. J Archaeol Sci 28:1247–1255. https://doi.org/10.1006/jasc.2001.0698
O’Keefe CE, Cadrin SX, Stokesbury KDE (2013) Evaluating effectiveness of time/area closures, quotas/caps, and fleet communications to reduce fisheries bycatch. ICES J Mar Sci 71:1286–1297. https://doi.org/10.1093/icesjms/fst063
Osorio F, Vallejos R, Cuevas F (2014) SpatialPack: package for analysis of spatial data. R package version 0.2–3
Pardo SA, Burgess KB, Teixeira D, Bennett MB (2015) Local-scale resource partitioning by stingrays on an intertidal flat. Mar Ecol Prog Ser 533:205–218. https://doi.org/10.3354/meps11358
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Polechová J, Storch D (2019) Ecological niche. In: Fath B (ed) Encyclopedia of Ecology (Second Edition). Elsevier Oxford, pp 72–80
Pontón-Cevallos J, Dwyer RG, Franklin CE, Bunce A (2017) Understanding resource partitioning in sympatric seabirds living in tropical marine environments. Emu Austr Ornithol 117:31–39. https://doi.org/10.1080/01584197.2016.1265431
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecol 83:703–718
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. https://doi.org/10.1007/s00442-006-0630-x
Prieto R, Janiger D, Silva MA, Waring GT, Gonçalves JM (2012) The forgotten whale: a bibliometric analysis and literature review of the North Atlantic sei whale Balaenoptera borealis. Mamm Rev 42:235–272. https://doi.org/10.1111/j.1365-2907.2011.00195.x
Quantum GIS (2017) Development Team (2014) QGIS Geographic Information System. Open Source Geospatial Foundation Project.
R Development Core Team (2003) The R Reference Manual: Base Package. Network Theory
Reeves RR, Smith TD, Josephson EA, Clapham PJ, Woolmer G (2004) Historical observations of humpback and blue whales in the north Atlantic ocean: clues to migratory routes and possibly additional feeding grounds. Mar Mamm Sci 20:774–786. https://doi.org/10.1111/j.1748-7692.2004.tb01192.x
Reisinger RR, Carpenter-Kling T, Connan M, Cherel Y, Pistorius PA (2020) Foraging behaviour and habitat-use drives niche segregation in sibling seabird species. R Soc Open Sci. https://doi.org/10.1098/rsos.200649
Reiss L, Häussermann V, Mayr C (2020) Stable isotope records of sei whale baleens from Chilean Patagonia as archives for feeding and migration behavior. Ecol Evol 10:808–818. https://doi.org/10.1002/ece3.5939
Rita D, Borrell A, Víkingsson G, Aguilar A (2019) Histological structure of baleen plates and its relevance to sampling for stable isotope studies. Mamm Biol 99:63–70. https://doi.org/10.1016/j.mambio.2019.10.004
Robertson GS, Bolton M, Grecian WJ, Wilson LJ, Davies W, Monaghan P (2014) Resource partitioning in three congeneric sympatrically breeding seabirds: foraging areas and prey utilization. Auk 131:434–446. https://doi.org/10.1642/auk-13-243.1
Robillard A, Gauthier G, Therrien J-F, Bêty J (2021) Linking winter habitat use, diet and reproduction in snowy owls using satellite tracking and stable isotope analyses. Isotopes Environ Health Stud 57:166–182. https://doi.org/10.1080/10256016.2020.1835888
Rocha RC Jr, Clapham PJ, Ivashchenko Y (2015) Emptying the oceans: a summary of industrial whaling catches in the 20th century. MFR 76:37–48. https://doi.org/10.7755/MFR.76.4.3
Rockwood RC, Elliott ML, Saenz B, Nur N, Jahncke J (2020) Modeling predator and prey hotspots: management implications of baleen whale co-occurrence with krill in Central California. PLoS ONE. https://doi.org/10.1371/journal.pone.0235603
Roman J, Estes JA, Morissette L, Smith C, Costa D, McCarthy J, Nation JB, Nicol S, Pershing A, Smetacek V (2014) Whales as marine ecosystem engineers. Front Ecol Environ 12:377–385
Roques L, Chekroun MD (2011) Probing chaos and biodiversity in a simple competition model. Ecol Complex 8:98–104. https://doi.org/10.1016/j.ecocom.2010.08.004
Ross ST (1986) Resource partitioning in fish assemblages: a review of field studies. Copeia 1986:352–388. https://doi.org/10.2307/1444996
Rowntree VJ, Valenzuela LO, Fraguas PF, Seger J (2008) Foraging behaviour of southern right whales (Eubalaena australis) inferred from variation of carbon stable isotope ratios in their baleen. International Whaling Commission annual meeting document SC/60/BRG23
RStudio Team (2020) RStudio: integrated development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol 19:256–263. https://doi.org/10.1016/j.tree.2004.03.017
Ryan C, McHugh B, Trueman CN, Sabin R, Deaville R, Harrod C, Berrow SD, O’Connor I (2013) Stable isotope analysis of baleen reveals resource partitioning among sympatric rorquals and population structure in fin whales. Mar Ecol Prog Ser 479:251–261. https://doi.org/10.3354/meps10231
Santora JA, Reiss CS, Loeb VJ, Veit RR (2010) Spatial association between hotspots of baleen whales and demographic patterns of Antarctic krill Euphausia superba suggests size-dependent predation. Mar Ecol Prog Ser 405:255–269. https://doi.org/10.3354/meps08513
Sasaki H, Murase H, Kiwada H, Matsuoka K, Mitani Y, Saitoh S-I (2013) Habitat differentiation between sei (Balaenoptera borealis) and Bryde’s whales (B. brydei) in the western North Pacific. Fish Oceanogr 22:496–508. https://doi.org/10.1111/fog.12037
Schoeninger MJ, DeNiro MJ (1984) Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta 48:625–639. https://doi.org/10.1016/0016-7037(84)90091-7
Segre PS, Weir CR, Stanworth A (2021) Biomechanically distinct filter-feeding behaviors distinguish sei whales as a functional intermediate and ecologically flexible species. J Exp Bio 224:238873
Seyboth E, Botta S, Mendes CRB, Negrete J, Dalla Rosa L, Secchi ER (2018) Isotopic evidence of the effect of warming on the northern Antarctic Peninsula ecosystem. Deep Sea Res Part 2 Top Stud Oceanogr. https://doi.org/10.1016/j.dsr2.2017.12.020
Shabangu FW, Findlay KP, Yemane D, Stafford KM, van den Berg M, Blows B, Andrew RK (2019) Seasonal occurrence and diel calling behaviour of Antarctic blue whales and fin whales in relation to environmental conditions off the west coast of South Africa. J Mar Syst 190:25–39. https://doi.org/10.1016/j.jmarsys.2018.11.002
Sheaves M, Sheaves J, Stegemann K, Molony B (2013) Resource partitioning and habitat-specific dietary plasticity of two estuarine sparid fishes increase food-web complexity. Mar Freshwater Res 65:114–123. https://doi.org/10.1071/MF12348
Silva MA, Borrell A, Prieto R, Gauffier P, Bérubé M, Palsbøl PJ, Colaço A (2019) Stable isotopes reveal winter feeding in different habitats in blue, fin and sei whales migrating through the Azores. Royal Soc Open Sci 6:181800
Targett TE (1978) Food resource partitioning by the pufferfishes Sphoeroides spengleri and S. testudineus from Biscayne Bay. Florida Mar Biol 49:83–91. https://doi.org/10.1007/bf00390732
Teixeira CR, Botta S, Daura-Jorge FG, Pereira LB, Newsome SD, Simões-Lopes PC (2021) Niche overlap and diet composition of three sympatric coastal dolphin species in the southwest Atlantic Ocean. Mar Mamm Sci 37:111–126. https://doi.org/10.1111/mms.12726
Tønnessen JN, Johnsen AO (1982) The History of Modern Whaling. University of California Press
Trathan PN, Reid K (2009) Exploitation of the marine ecosystem in the sub-Antarctic: historical impacts and current consequences. Pap Proc R Soc Tasman 143:9–14
Trathan PN, Forcada J, Murphy EJ (2007) Environmental forcing and Southern Ocean marine predator populations: effects of climate change and variability. Philos Trans R Soc Lond B Biol Sci 362:2351–2365. https://doi.org/10.1098/rstb.2006.1953
Trathan PN, Ratcliffe N, Masden EA (2012) Ecological drivers of change at South Georgia: the krill surplus, or climate variability. Ecography 35:983–993. https://doi.org/10.1111/j.1600-0587.2012.07330.x
Trathan PN, Collins MA, Grant SM, Belchier M, Barnes DKA, Brown J, Staniland IJ (2014) Chapter Two - The South Georgia and the South Sandwich Islands MPA: Protecting A Biodiverse Oceanic Island Chain Situated in the Flow of the Antarctic Circumpolar Current. In: Johnson ML, Sandell J (eds) Advances in Marine Biology. Academic Press, pp 15–78
Trueman CN, Jackson AL, Chadwick KS, Coombs EJ, Feyrer LJ, Magozzi S, Sabin RC, Cooper N (2019) Combining simulation modeling and stable isotope analyses to reconstruct the last known movements of one of Nature’s giants. PeerJ. https://doi.org/10.7717/peerj.7912
Valenzuela LO, Rowntree VJ, Sironi M, Seger J (2018) Stable isotopes (δ15N, δ13C, δ34S) in skin reveal diverse food sources used by southern right whales Eubalaena australis. Mar Ecol Prog Ser 603:243–255. https://doi.org/10.3354/meps12722
Visser ME, Both C (2005) Shifts in phenology due to global climate change: the need for a yardstick. Proc Biol Sci 272:2561–2569. https://doi.org/10.1098/rspb.2005.3356
Votier SC, Bearhop S, MacCormick A, Ratcliffe N, Furness RW (2003) Assessing the diet of great skuas, Catharacta skua, using five different techniques. Polar Biol 26:20–26
Wada E, Mizutani H, Minagawa M (1991) The use of stable isotopes for food web analysis. Crit Rev Food Sci Nutr 30:361–371. https://doi.org/10.1080/10408399109527547
Waluda CM, Hill SL, Peat HJ, Trathan PN (2017) Long-term variability in the diet and reproductive performance of penguins at Bird Island. Mar Biol, South Georgia. https://doi.org/10.1007/s00227-016-3067-8
Weir CR, Oms G, Baracho-Neto CG, Wedekin LL, Daura-Jorge FG (2020) Migratory movement of a sei whale ( Balaenoptera borealis ) between Brazil and the Falkland Islands (Malvinas). Mar Mamm Sci 36:1050–1057. https://doi.org/10.1111/mms.12687
West JB, Bowen GJ, Cerling TE, Ehleringer JR (2006) Stable isotopes as one of nature’s ecological recorders. Trends Ecol Evol 21:408–414. https://doi.org/10.1016/j.tree.2006.04.002
Witteveen BH, Wynne KM (2016) Trophic niche partitioning and diet composition of sympatric fin (Balaenoptera physalus) and humpback whales (Megaptera novaeangliae) in the Gulf of Alaska revealed through stable isotope analysis. Mar Mamm Sci 32:1319–1339
Zenteno L, Borella F, Otero JG, Piana E, Belardi JB, Borrero LA, Saporiti F, Cardona L, Crespo E (2015) Shifting niches of marine predators due to human exploitation: the diet of the South American sea lion (Otaria flavescens) since the late Holocene as a case study. Paleobiology 41:387–401. https://doi.org/10.1017/pab.2015.9
Zerbini AN, Andriolo A, Heide-Jørgensen MP, Pizzorno JL, Maia YG, VanBlaricom GR, DeMaster DP, Simões-Lopes PC, Moreira S, Bethlem C (2006) Satellite-monitored movements of humpback whales Megaptera novaeangliae in the Southwest Atlantic Ocean. Mar Ecol Prog Ser 313:295–304. https://doi.org/10.3354/meps313295
Acknowledgements
The authors thank David Walton, Bob Burton and Bob Headland for sharing baleen samples that they collected at South Georgia, Catherine Kneale for advice on laboratory protocols and assistance performing isotope ratio mass spectrometry, James Rolfe and the Godwin Laboratory for Palaeoclimate Research (Dept. of Earth Sciences, University of Cambridge) for facilitation of isotope ratio mass spectrometry. The authors are grateful to Billy Mills and Matt Page for their helpful comments, which helped to significantly improve upon earlier versions of this manuscript, and to Alberto Marcias for providing the graphical representations of fin and sei whales used in the figures. D.L.B is supported by a NERC-Cambridge ESS Doctoral Training Partnership studentship from the Natural Environment Research Council (NE/L002507/1). Stable isotope analyses were funded by the Dorothy Garrod Laboratory at the McDonald Institute of Archaeological Research, Cambridge. This work represents a contribution to the Ecosystems component of the British Antarctic Survey Polar Science for Planet Earth Programme, funded by the Natural Environment Research Council.
Funding
This research was supported by D.L.B and a NERC-Cambridge ESS Doctoral Training Partnership studentship from the Natural Environment Research Council (NE/L002507/1). Stable isotope analyses were funded by the Dorothy Garrod Laboratory at the McDonald Institute of Archaeological Research, Cambridge.
Author information
Authors and Affiliations
Contributions
DLB, JAJ, TCO and AM contributed to study concepts and design. DLB, EH and RHYL performed laboratory analysis and data collection. DLB performed the analysis, prepared the figures and wrote the manuscript. JAJ contributed to manuscript preparation. All authors critically reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study because all experimental work was conducted on whale baleen material that was discarded at sites in South Georgia during the commercial whaling era. Material was transported to the UK by individual researchers prior to the international agreement for CITES regulation of shipment of whales. CITES was adopted by the UK in 1976, and whales were listed under Appendix I of CITES between 1975 and 1986.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buss, D.L., Hearne, E., Loy, R.H.Y. et al. Evidence of resource partitioning between fin and sei whales during the twentieth-century whaling period. Mar Biol 169, 150 (2022). https://doi.org/10.1007/s00227-022-04131-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04131-x