Abstract
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin’s lymphoma with increasing prevalence. Although the disease burden associated with DLBCL is high, only limited data on healthcare resource utilization (HCRU) and associated costs of German patients with DLBCL is available.
Methods
Using a large claims database of the German statutory health insurance with 6.7 million enrollees, we identified patients who were newly diagnosed with DLBCL between 2011 and 2018 (index date). Treatment lines were identified based on a predefined set of medication. HCRU and related costs were collected for the entire post index period and per treatment line.
Results
A total of 2495 incident DLBCL patients were eligible for the analysis. The average follow-up time after index was 41.7 months. During follow-up, 1991 patients started a first-line treatment, 868 a second-line treatment, and 354 a third-line treatment. Overall, patients spent on average (SD) 5.24 (6.17) days per month in hospital after index. While on anti-cancer treatment, this number increased to nine (10.9) in first-line, 8.7 (13.7) in second-line, and 9.4 (15.8) in third-line treatments. Overall costs per patient per month (PPPM) increased from €421 (875.70) before to €3695 (4652) after index. While on a treatment line, PPPM costs were €17,170 (10,246) in first-line, €13,362 (12,685) in second-line, and €12,112 (16,173) in third-line treatments. Time-unadjusted absolute costs sum up to €59,868 (43,331), €35,870 (37,387), and €28,832 (40,540) during first-line, second-line, and third-line treatments, respectively. The main cost drivers were hospitalizations (71% of total costs) and drug acquisition costs (18% of total costs).
Conclusions
The financial burden of DLBCL in Germany is high, mainly due to hospitalization and drug costs. Therefore, there is a high medical need for new cost-effective therapeutic options that can lower the disease burden and remain financially viable to support the growing number of patients with this aggressive disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This real-world health economic study reports the financial burden of newly diagnosed patients with diffuse large B-cell lymphoma (DLBCL) in Germany. |
Average cost after diagnosis is €3695 per patient per month. |
While on a treatment line, per-patient per-month costs increase to €17,170 in first-line, €13,362 in second-line, and €12,112 in third-line treatments. |
Compared to the USA, costs in Germany are up to four times lower. |
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin’s lymphoma (NHL) and is associated with a 5-year mortality of 40–50% [1]. The yearly incidence in Germany is 5333, which corresponds to 6.6 newly diagnosed patients per 100,000 inhabitants [2]. Incidence rates are on the rise in Europe and US, mainly due to the ageing of the population [3]. However, data on resource utilization and associated costs is scarce for Germany. One notable exception is a recent retrospective single-center observational study of a large German tertiary teaching hospital [4]. The study focused on 84 relapsed or refractory patients with at least one prior treatment line. Mean number of days of hospital stay were 44 days for second-line therapy, 26 days for third-line therapy, and 63 days for subsequent lines. Average cost per patient was €44,750 in the second, €32,589 in the third, and €88,668 in later lines of treatment. Tertiary teaching hospitals on the other hand are not necessarily representative for the entire healthcare system. In Germany, many cancer patients are treated in the outpatient setting in specialized oncology practices. Evidence suggests that hematological malignancies can be treated in an outpatient setting that goes along with a reduction of hospital admission without compromising on clinical efficacy [5, 6]. For the US, it was reported that hospital-based cancer treatment was almost 60% more expensive than in the community setting [7]. However, due to institutional differences between the healthcare systems of the USA and Germany, those findings are not generalizable. For this reason, a fresh look at Germany is warranted. To get a more comprehensive picture of health utilization and associated treatment costs of DLBCL patients in Germany, we analyzed a large German claims database that is representative of the German population that contained data both from hospitals and from the primary care sector.
Methods
Data Source and Sample Size
De-identified records were obtained from a claims database provided by Team Gesundheit GmbH, Essen, Germany. The database contains detailed electronic records of health insurance claim information on inpatient, outpatient, and prescription drug data at the individual member level. The database covers 6.7 million persons of the German statutory health insurance between 2010 and 2019. All individual patient data are anonymized in the research database to comply with German data protection regulations. Therefore, institutional review board and ethical approval and informed consent of the individuals were not required. These data originated from different company health insurance funds (Betriebskrankenkassen), which are part of the German statutory health insurance scheme. Membership in the statutory health insurance scheme is compulsory for 88% of the German population. The remaining 12% of the population is privately insured. The database has been used for health services research covering multiple indications [8,9,10].
Study Design and Patient Selection
Patients were identified and selected for participating in this study using ICD-10-CM diagnoses codes for DLBCL (C83.3). The cohort of patients is identified using outpatient and/ or inpatient care data. To be selected patients had at least one main or secondary diagnosis of DLBCL in inpatient data or at least two different diagnoses of DLBCL in two different quarters within 1 year in outpatient data (M2Q criterion). The index quarter was defined as the quarter of the first diagnosis of DLBCL. Patients were considered as incident, if they had no other diagnosis of C83.3 within four quarters before the index (look back period). The study period encompassed at least four quarters pre-index and at least four quarters post-index, including the index quarter. Patients must have been continuously enrolled for at least eight quarters including four quarters before the index unless death from any cause occurred within this period (see Fig. 1 for a graphical description of the study design).
Exclusion criteria for the study were presence of other cancer diagnosis during the pre-index period that are listed in Supplementary Table 1 (see Table 1).
To identify a therapy regimen that consists of a combination of different compounds or treatments, we applied the approach by Tsutsue et al. [11]. We first considered a set of treatments that are frequently used for the treatment of DLBCL (Table 2). All drugs that were added within 30 days after treatment initiation are considered as part of the same regimen and line of therapy. All treatments of interest which are documented during this time slot beginning with the date of the first prescription will be summarized to a therapy line. This therapy line is ended if a new drug is added after the first 30 days of treatment initiation. The end date of the therapy line is then defined as the day before the prescription date of the new drug. Furthermore, a therapy line is ended if a treatment gap of 90 days is observed. In this case, the end of the last therapy line will be defined as the date of the last prescription plus 21 days [12].
Besides drug treatment, we also report how many DLBCL patients received either autologous or allogeneic stem cell transplantation (SCT) or radiotherapy during their treatment. This will be reported both overall and per therapy line. The patient cohort will also be described by demographic parameters such as age and sex at index. Comorbidities were measured by means of the Charlson Comorbidity Index (CCI). The ICD-10 coding algorithms for Charlson comorbidities were made available by Quan et al. [13]. In DLBCL, CCI scores above 2 were shown to be associated with a significantly higher mortality [14]. Among first-line patients receiving R-CHOP regimen, 3-year survival rate was 39% for those with a CCI score ≥ 2 while the respective value for patients with a CCI of 0 or 1 was twice as high (81%) [15].
Healthcare Resource Utilization (HCRU) and Associated Costs
Healthcare resource utilization (HCRU) and associated costs will be analyzed during pre-index (four quarters) and post-index time period (index quarter to time to end of follow-up). We look at the following categories: Hospitalizations, outpatient office visits, drug prescriptions, sick leave, other items such as stationary rehabilitation, travel expenses, remedies, and medical aids.
The costs are allocated to the time periods and therapy lines based on their respective billing dates. In addition to this, hospital treatments and stationary rehabilitation are allocated based on their discharge dates and travel expenses, and remedies and aids are allocated based on their beginning dates. The costs of sickness benefits during a therapy line are determined analogous to the payment to the insured. The payment days during a therapy line are multiplied by the daily payment amount to calculate the sickness benefit costs of a patient during a therapy line.
The different costs are calculated per patient and month both for the total time period after index and per therapy line. For this purpose, the sum of costs per patient and period respectively per patient and therapy line are divided by the duration in months of the corresponding period or therapy line to report costs per patient per month (PPPM). In addition, we report total costs per therapy line (i.e., without time adjustment) in order to ensure comparability with international studies.
Data Analysis
Categorical variables are presented as the count and percentage of patients. Continuous variables are summarized by providing the mean and standard deviation (see Fig. 2).
Results
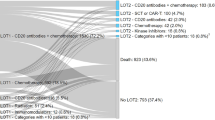
Among 6.7 million database enrollees, we identified 3840 incident DLBCL patients who were continuously enrolled in the database; 2495 of them had no other cancer diagnosis and were selected for the analysis. Of note, for 20% of the selected patients, no DLBCL-related pharmaceutical intervention as defined in Table 2 was detected (Fig. 1).
The average (median) follow-up time after index was 41.7 (33.5) months for the overall cohort.
Patient characteristics are reported in Table 3. The majority (58%) of the cohort were male, mean (median) age at index date was 67 (71). Pre index CCI score was 4.4, indicating a rather morbid population.
Healthcare Resource Utilization
HCRU is reported in Table 4. After initial diagnosis of DLBCL the monthly HCRU is multiplying. The number of hospital admissions increased from 0.06 to 0.4 (+ 566%) and days spent in the hospital from 0.57 to 5.24 (+ 819%). Including only those patients with at least one treatment line, post index number of monthly days in hospital was 4.9. While on anti-cancer treatment, this number increased to 9 in first-line, 8.7 in second-line, and 9.4 in third-line treatments. Overall, patients who received no treatment according to our definition spend on average more days per month in hospital (6.73, SD 7.61) than those with anti-cancer treatment (4.87, SD 5.68).
Total days spent in hospital can also be calculated when the length of treatment line is taken into consideration. During first-line treatment, the total days spent in hospital was 28. For second-line treatment, the corresponding value was 22 days and for third-line treatment it was 21 days.
When it comes to resident doctor visits, patients had 1.98 monthly visits before their initial diagnosis. After index, this number increased to 3.66 for the overall cohort, to 2.87 for patients without treatment, and 3.87 for patients with at least one line of treatment. During anti-cancer treatment, those numbers were even higher. Monthly days on sick leave were 0.67 before index and 2.20 thereafter for the overall cohort, 0.76 for those not receiving treatment and 2.57 for patients with anti-cancer treatment. While on treatment, those numbers increased to 6.7 (first-line), 4.9 (second-line), and 2.9 (third-line). Use of devices and stationary rehabilitation also increased after diagnosis. Furthermore, our analysis reveals that 4.9% of the overall cohort receives a stem cell transplantation. Radiation is more common (23.6% of the overall patient population, 10.1% of the patients without regimen, and 27.0% of patients receiving at least one regimen. While on a treatment line, the share of patients receiving radiation is much smaller, indicating that radiation is prescribed in between therapy lines.
Costs
PPPM costs are reported in Table 5. During total follow-up time, acquisition costs for drugs increase from €112 per month to €784 for those who received at least one treatment. During treatment lines, drug costs are higher, namely €3345 per month for first-line, €2255 for second-line, and €1610 for third-line treatments.
Hospitalization costs do increase at even at a higher rate. Pre index PPPM cost was €182, which goes up to €2618 for the overall population, and is even higher for people receiving no treatment (€3888) during total follow-up, representing an increase of 2036%. For those receiving at least one regimen, costs are €2297. While on a treatment line, hospitalization costs are €4362 in the first, €3698 in the second, and €3831 in the third-line treatments. Outpatient and other costs (e.g., for rehabilitation) do increase as well after index but are on a much smaller scale than hospitalization costs. Sickness leave costs were very small before index (€15) and increase to €333 in first, €264 in second, and €149 in third-line treatments. Overall costs were €421 per month and go up to €3695 for the overall cohort for total follow-up, €4393 for those without pharmaceutical treatment according to the predefined criteria, and €3518 for patients with at least one therapy. A graphical summary of the pre- and post-index costs for the overall cohort is given in Fig. 3.
Figure 4 presents the share of each cost component on total post index costs for the overall cohort on a PPPM basis; 71% of total costs are due to hospitalizations, followed by drugs costs (18%). The remaining costs are all below 5% each.
Total costs per treatment line that are not time-adjusted are displayed in Fig. 5. During the first-, second-, and third-line treatments, the absolute amounts are €59,868, €35,870, and €28,832, respectively (Fig. 5).
Discussion
Despite of the increasing incidence of DLBCL in Germany, data on cost and resource utilization are still limited. To the best of our knowledge, this attempt to calculate the financial burden of DLBCL patients was the first analysis that draws on a dataset which is representative of the German population. In our analysis, PPPM costs were €421 before the first DLBCL diagnosis and € 3695 thereafter, indicating an almost ninefold increase in costs. Turning to the time-unadjusted total costs of the first-, second-, and third-line treatments, the amounts were €59,868, €35,870, and €28,832, respectively. The costs are slightly smaller compared to Moertl et al. [4] who analyzed costs in a tertiary teaching hospital setting while our data provide a picture of both the hospital and the primary care sector. They reported second-line costs of €44,750, and third-line costs of €32,589. Lower costs in our analysis are mainly due to lower hospital utilization, in particular in second-line treatment. While Moertl et al. reported mean number of hospital length of stay of 44 days for second-line therapy, and 26 days for third-line therapy, the corresponding values in our analysis were 22 and 21 respectively. A possible explanation for this observation is that the median age in our representative sample is quite high (71 years). Elderly patients are usually not eligible for autologous stem cell transplantation, which is the recommended treatment in second-line treatment. In comparison, the median age in Moertl et al. was only 61 when SCT is still the preferred treatment option. Therefore, the share of patients treated with (costly) SCT is much higher in their analysis compared with the overall patient population.
On an international scale, costs seem to be much higher in the US according to a recent study covering the timespan between 2011 and 2017 [16]. Mean total healthcare expenditures (treatment duration) for first-, second-, and third-line treatments were $111,314 (124.5 days), $88,472 (80.8 days), and $103,365 (70.9 days), respectively. While the treatment duration is very close to our findings, associated costs are much higher, especially in later lines of therapy. Another US study reported average annual costs of $137,156 across therapy lines for patients receiving treatment [17]. Another study concluded that average yearly costs for second-line patients were even higher, and stood at $210,488 [18]. On a PPPM basis, estimates for the US range from $11,900 [19] to $15,600 [20] compared with €3695 in our study. Taking exchange rates of 2018, this amount translates to $4250. The main reason for the huge cost differences between Germany and the US are institutional differences in health care financing. The health care market in the United States is largely unregulated in terms of prices, which results in higher costs for both drugs and medical services [21, 22]. Compared with Japan, which is more similar to Germany in terms of the health care system, costs are higher in Germany. Tsutsué et al. [11] reported costs during first-, second-, and third-line treatments of $37,205, $26,248, and $23,354, respectively. Those findings support the popular view that the Japanese health care system allocates resources very efficiently [23]. Results from the UK indicate lower costs compared to the US as well. For curative chemotherapy, the predicted medical costs were £14,966, £23,449, and £7376 for first-, second-, and third-line treatments, respectively [24].
We find that the lion share of the total costs goes for hospitalizations (70%), a finding that is well supported by the literature [11]. However, the cost share incurred by outpatient office visits is only 4% in our analysis, which is substantially lower than reported in other studies [25].
Limitations
Several limitations of the study should be acknowledged. Claims data in general are not designed for research purposes and offer only a very limited set of medical parameters. A common problem in claims database research is the coding quality, as disease codes do not always reflect clinical reality [26, 27]. A recent study for instance estimates that only less than 50% of patients with a suspected chronic graft versus host disease have a documented diagnosis [28]. Moreover, treatments that are not financed by the health insurance are not recorded. This could be a potential source of a bias because some patients with no record of cancer-specific therapies might have been enrolled in a clinical trial. In that case, medication is financed by the study sponsor and is not visible in the database.
Conclusions
The financial burden of DLBCL in Germany is high, mainly due to hospitalization and drug costs. This constitutes a high need for new cost-effective therapeutic options that can lower the disease burden and remain financially viable to support the growing number of patients with this aggressive disease. Comparing our results with other studies, our results are plausible and can potentially be used as input parameters for health economic evaluations.
References
Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–8.
Gemeinsamer Bundesauschuss. Nutzenbewertungsverfahren zum Wirkstoff Polatuzumab Vedotin, 2020, available: https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/518/#dossier
Kanas G, Ge W, Quek R, et al. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020–2025. Leuk Lymphoma. 2022;63(1):54–63.
Moertl B, Dreyling M, Schmidt C at al. Inpatient treatment of relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL): a health economic perspective. Clin Lymphoma Myeloma Leuk. 2022,22(7):474–482.
Daly AB, Cuthbert R, Finnegan D, et al. A comparison of inpatient and outpatient-based chemotherapy regimens for the treatment of acute myeloid leukaemia in the elderly. Ulster Med J. 2019;88(1):25–9.
Egerer G, Hegenbart U, Salwender H, et al. Outpatient treatment of multiple myeloma with a combination of vincristine. Adriamycin and dexamethasone Support Care Cancer. 2001;9:380–5.
Gordan L, Blazer M, Saundankar V et al. Cost Differences associated with oncology care delivered in a community setting versus a hospital setting: a matched-claims analysis of patients with breast, colorectal, and lung cancers. J Oncol Pract 2018:JOP1700040.
Bögemann M, Zagorska A, Akumo D, et al. Using data from a sickness fund claims database to assess the treatment patterns and healthcare resource utilization among patients with metastatic renal cell carcinoma in Germany. Urol Int. 2020;104(11–12):982–93.
Mahlich J, Olbrich K, Wilk A, et al. Time to treatment discontinuation in German patients with schizophrenia: long-acting injectables versus oral antipsychotics. Clin Drug Investig. 2021;41(1):99–113.
Mahlich J, Alba A, El Hadad L, et al. Drug survival of biological therapies for psoriasis treatment in Germany and associated costs: a retrospective claims database analysis. Adv Ther. 2019;36(7):1684–99.
Tsutsué S, Tobinai K, Yi J, et al. Nationwide claims database analysis of treatment patterns, costs and survival of Japanese patients with diffuse large B-cell lymphoma. PLoS ONE. 2020;15(8): e0237509.
Armitage J. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110(1):29–36.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Kobayashi Y, Miura K, Hojo A, et al. Charlson Comorbidity Index is an independent prognostic factor among elderly patients with diffuse large B-cell lymphoma. J Cancer Res Clin Oncol. 2011;137:1079–84.
Kocher F, Mian M, Seeber A, et al. The prognostic impact of comorbidities in patients with de-novo diffuse large B-cell lymphoma treated with R-CHOP immunochemotherapy in curative intent. J Clin Med. 2020;9(4):1005.
Tkacz J, Garcia J, Gitlin M, et al. The economic burden to payers of patients with diffuse large B-cell lymphoma during the treatment period by line of therapy. Leuk Lymphoma. 2020;61(7):1601–9.
Yang X, Laliberté F, Germain G et al. Real-world characteristics, treatment patterns, health care resource use, and costs of patients with diffuse large B-cell lymphoma in the U.S. Oncologist. 2021;26(5):e817-e826.
Purdum A, Tieu R, Reddy SR, et al. Direct costs associated with relapsed diffuse large B-cell lymphoma therapies. Oncologist. 2019;24(9):1229–36.
Morrison VA, Bell JA, Hamilton L, et al. Economic burden of patients with diffuse large B-cell and follicular lymphoma treated in the USA. Future Oncology 2018 10;14(25):2627–2642.
Ren J, Asche CV, Shou Y, et al. Economic burden and treatment patterns for patients with diffuse large B-cell lymphoma and follicular lymphoma in the USA. Journal of Comparative Effectiveness Research 2019 4;8(6):393–402
Danzon PM, Chao L-W. Cross-national price differences for pharmaceuticals: how large, and why? J Health Econ. 2000;19:159–95.
Laugesen M, Glied S. Higher fees paid to US physicians drive higher spending for physician services compared to other countries. Health Aff (Millwood). 2011;30:1647–56.
Rump A, Schöffski O. The German and Japanese health care systems: an international comparison using an input-output model. Public Health. 2016;141:63–73.
Wang HI, Smith A, Aas E, et al. Treatment cost and life expectancy of diffuse large B-cell lymphoma (DLBCL): a discrete event simulation model on a UK population-based observational cohort. Eur J Health Econ. 2017;18(2):255–67.
Huntington S, Keshishian A, McGuire M, et al. Costs of relapsed diffuse large B-cell lymphoma among Medicare patients. Leuk Lymphoma. 2018;59(12):2880–7.
Czwikla J, Domhoff D, Giersiepen K. ICD coding quality for outpatient cancer diagnoses in SHI claims data. Z Evid Fortbild Qual Gesundh wesen. 2016;118:48–55.
Scheid C, Kudernatsch R, Eckart M, et al. Incidence of graft-versus-host-disease in Germany: evidence from health care claims data. J Public Health. 2022. https://doi.org/10.1007/s10389-022-01736-w.
Scheid C, Kudernatsch R, Eckart M, et al. Treatment pathways and health outcomes of German patients with chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation: a retrospective health claims data analysis. Drugs - Real World Outcomes. 2022. https://doi.org/10.1007/s40801-022-00320-8.
Acknowledgements
Funding
This study, and the journal’s Rapid Service fee, were funded by Miltenyi Biomedicine GmbH.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Peter Borchmann, Jörg Mahlich, Michael Papadimitrious, Sybille Riou, and Barbara Werner designed the study. Jörg Mahlich drafted the manuscript and Barbara Werner did the statistical analysis. Peter Borchmann and Jan-Michel Heger revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Disclosures
Jörg Mahlich, Sybille Riou, and Michael Papadimitrious are employees of Miltenyi Biomedicine GmbH. Barbara Werner is an employee of Team Gesundheit, a contract research company that received funding from Miltenyi Biomedicine to conduct the study in line with the study protocol. Peter Borchmann and Jan-Michel Heger are employed at the University Hospital Cologne. All authors have no other relevant affiliations or financial involvement with any other organization or entity with a financial interest in the subject matter or materials discussed in the manuscript apart from those disclosed.
Compliance with Ethics Guidelines
All individual patient data are anonymized in the research database to comply with German data protection regulations. Institutional review board/ethical approval and informed consent of the individuals were not required. Team Gesundheit owns the database used in this study and the necessary permissions were received for its usage.
Data Availability
Due to the sensitivity of the data and data protection regulations, the analysis datasets of the current study cannot not be shared or stored at a public repository.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Borchmann, P., Heger, JM., Mahlich, J. et al. Healthcare Resource Utilization and Associated Costs of German Patients with Diffuse Large B-Cell Lymphoma: A Retrospective Health Claims Data Analysis. Oncol Ther 11, 65–81 (2023). https://doi.org/10.1007/s40487-022-00211-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-022-00211-6