Abstract
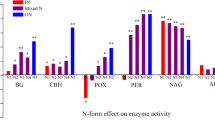
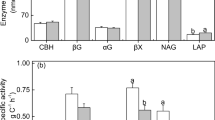
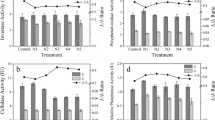
The effect of different inputs of mineral N on several enzyme activities involved in the C and N cycles was investigated using Oa material of forest floors from four Norway spruce [Picea abies (L.) Karst.] sites with different C-to-N ratios. The samples from each site were treated with five different concentrations of mineral N (as liquid NH4NO3). All samples were incubated aerobically for 15–20 weeks at 15°C and at field capacity. Respiration was measured weekly. At the end of the incubation period, four enzyme activities (endoglucanase, β-glucosidase, polyphenol oxidase and β-glucosaminidase) and microbial biomass were determined. Endoglucanase activity was increased and β-glucosidase activity was decreased by N additions only in Oa material having a wide C-to-N ratio. In N-supplemented samples of low C-to-N ratio, increased polyphenol oxidase activities were often detected as a consequence of N addition. β-Glucosaminidase activity responded positively to mineral N additions, particularly in Oa samples with low internal N concentration. The results of the present study indicate that the effects of N additions on enzymatic activities of organic matter in late stages of decomposition are related to the C-to-N ratio. Increasing inputs of mineral N to spruce ecosystems may especially affect C-hydrolyzing enzyme activities in soils with wide C-to-N ratio leading to an incomplete degradation of cellulose and thus reduced C availability to micro-organisms.


Similar content being viewed by others
References
Ajwa HA, Dell CJ, Rice CW (1999) Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 31:769–777
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Anderson JPE, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manage 133:13–22
Berg B, Matzner E (1997) Effect of N deposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DE (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Chunderova AN (1970) Enzyme activity and pH of soil. Sov Soil Sci 5:308–314
Dick RP (1994) Soil enzyme activities as indicators of soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment. (SSSA special publication 35) Soil Science Society of America, Madison, Wis., pp 107–124
Dill I, Kraepelin G (1988): Der Abbau von Lignin/Cellulose durch Weißfäule-Pilze: Einfluß spezifischer ökologischer Faktoren. Forum Mikrobiol 11/88:484–489
Dilly O, Nannipieri P (1998) Intracellular and extracellular enzyme activity in soil with reference to elemental cycling. Z Planzenernaehr Bodenkd 161:243–248
Dunger W, Fiedler HJ (1997) Methoden der Bodenbiologie, 2nd edn. Fischer, Jena
Eivazi F, Tabatabai MA (1990) Factors affecting glucosidase and galactosidase activities in soils. Soil Biol Biochem 22:891–897
Eriksson K-E, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer, Berlin Heidelberg New York
Fengel D, Wegener G (1984) Wood: chemistry, ultrastructure, reactions. de Gruyter, Berlin
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Gong CS, Tsao GT (1979) Cellulase and biosynthesis regulation. Annu Rep Ferm Proc 3:111-140
Haider K (1999) Von der toten organischen Substanz zum Humus. Z Planzenernaehr Bodenkd 162:363–371
Henriksen TM, Breland TA (1999) Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol Biochem 31:1121–1134
Hoffmann G, Dedeken M (1965) Eine Methode zur kolorimetrischen Bestimmung der β-Glucosidaseaktivität in Böden. Z Planzenernaehr Bodenkd 108:195–201
Keyser P, Kirk TK, Zeikus JG (1978) Lignolytic enzyme system of Phanerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol 135:790–797
Kilham K (1994) Soil ecology. University Press, Cambridge
Kostov O, Petkova G, Van Cleemput O (1994) Microbial indicators for sawdust and bark compost stability and humification processes. Bioresource Technol 50:193–200
Leatham GF, Kirk TK (1983) Regulation of lignolytic activity by nutrient nitrogen in white-rot basidiomycetes. FEMS Microbiol Lett 16:65–67
McClaugherty C, Berg B (1987) Cellulose, lignin and nitrogen concentrations as rate regulating factors in late stages of forest litter decomposition. Pedobiologia 30:101–112
McLatchey GP, Reddy KR (1998) Regulation of organic matter decomposition and nutrient release in a wetland soil. J Environ Qual 27:1268–1274
Michel K (2002) Nitrogen as a factor of soil organic matter stability in forest soils. Bayreuther Forum Ökol 98:1–114
Michel K, Matzner E (2002) Nitrogen content of forest floor Oa layers affect carbon pathways and nitrogen mineralization. Soil Biol Biochem 34:1807–1813
Michlin DM, Bronovickaja ZS (1949) Iodometriceskij metod opredelenija polifenoloksidazy i peroksidazy. Biochimija 14:379–381
Miller M, Palojärvi A, Rangger A, Reeslev M, Kjøller A (1998) The use of fluorgenic substrates to measure fungal presence and activity in soil. Appl Environ Microbiol 64:613–617
Moorhead DL, Sinsabaugh RL (2000) Simulated patterns of litter decay predict patterns of extracellular enzyme activities. Appl Soil Ecol 14:71–79
Nannipieri P, Ceccanti B, Grego S (1990) Ecological significance of the biological activity in soil. In: Bollag J-M, Stotzky G (eds) Soil biochemistry, vol 6. Dekker, New York, pp 293–355
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick RP (eds) Enzymes in the environment. Dekker, New York, pp 1–33
Ohtonen R (1994) Accumulation of organic matter along a pollution gradient: application of Odum's theory of ecosystem energetics. Microbial Ecol 27:27–43
Ohtonen R, Markkola AM (1991) Biological activity and amount of FDA mycelium in mor humus of Scots pine stands (Pinus sylvestris L.) in relation to soil properties and degree of pollution. Biogeochemistry 13:1–26
Ohtonen R, Lähdesmäki P, Markkola AM (1994) Cellulase activity in forest humus along an industrial pollution gradient in Oulu, Northern Finland. Soil Biol Biochem 26:97–101
Parham JA, Deng SP (2000) Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem 32:1183–1190
Périé FH, Gold MH (1991) Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol 57:2240–2245
Persson T, Wiren A (1989) Microbial activity in forest soils in relation to acid/base and carbon/nitrogen status. Medd Nor Inst Skogforskning 42:83–94
Sakamoto K, Oba Y (1994) Effect of fungal to bacterial biomass ratio on the relationship between CO2 evolution and total soil microbial biomass. Biol Fertil Soils 17:39–44
Schinner F, von Mersi W (1990) Xylanase-, CM-cellulase- and invertase activity in soil: an improved method. Soil Biol Biochem 22:511–515
Sinsabaugh RL, Antibus K, Linkins AE, McClaugherty CA, Rayburn L, Repert D, Weiland T (1993) Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74:1586–1593
Skujins J (1978) History of abiotic soil enzyme research. In: Burns RG (ed) Soil enzymes. Academic Press, London, pp 1–49
Söderström B, Bååth E, Lundgren B (1983) Decrease in soil microbial activity and biomass owing to nitrogen amendments. Can J Microbiol 29:1500–1506
Stevenson FJ (1994) Humus chemistry, 2nd edn. Wiley, New York
Strobl W, Traunmüller M (1993) Bestimmung der β-Glucosidase-Aktivität. In: Schinner F, Öhlinger R, Kandeler E, Margesin R (eds) Bodenbiologische Arbeitsmethoden, 2nd edn. Springer, Berlin Heidelberg New York, pp 128–130
Tate RL (2000) Soil microbiology, 2nd edn. Wiley, New York
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Waldrop MP, Balser TC, Firestone MK (2000) Linking microbial community composition to function in a tropical soil. Soil Biol Biochem 32:1837–1846
Zaman M, Di HJ, Cameron KC, Frampton CM (1999) Gross nitrogen mineralization and nitrification rates and their relationships to enzyme activities and the microbial biomass in soils treated with dairy shed effluent and ammonium fertilizer at different water potentials. Biol Fertil Soils 29:178–186
Acknowledgements
We thank Alexandra Michel and Uwe Hell for help during the sampling, the Bavarian Forest Authorities for their support and Dr. Gunther Ilgen, Petra Dietrich and Kerstin Moser from the BITÖK central analytical department for C and N measurements. This research was funded by the Deutsche Forschungsgemeinschaft (DFG) and the German Ministry of Education, Science, Research and Technology (BMBF) under grant no. PT BEO-51–0339476.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michel, K., Matzner, E. Response of enzyme activities to nitrogen addition in forest floors of different C-to-N ratios. Biol Fertil Soils 38, 102–109 (2003). https://doi.org/10.1007/s00374-003-0622-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-003-0622-5