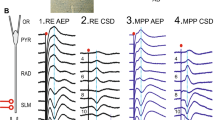
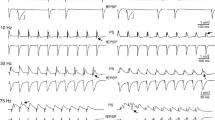
It is suggested that information on new stimuli from the neocortex is transmitted to the hippocampus, where temporal traces persist in the form of mosaics of modified synapses. During sleep, populations of neurons storing these traces are reactivated and return the information required for consolidation of a permanent memory trace to the neocortex. A possible mechanism for the reactivation of “trained” hippocampal neurons during memory consolidation consists of the reverberation of excitation in the neuron circuits linking the hippocampus and entorhinal cortex. Our studies in rats included recording of responses in hippocampal field CA1 to stimulation of Schaffer collaterals with potentiated synapses during waking and sleep. During deep sleep, discharges of field CA1 neurons were followed by waves of excitation which passed through the entorhinal cortex and reached the hippocampus and dentate gyrus via fibers of the perforant path, evoking neuron discharges in the latter. Repeated neuron discharges in field CA1 occurred on interaction of the early excitation wave returning directly via perforant path fibers and the late wave returning via Schaffer collaterals, not via the trisynaptic path via the dentate gyrus and hippocampal field CA3 but probably via field CA2.
Similar content being viewed by others
References
V. A. Zosimovskii and V. A. Korshunov, “Return to the hippocampal formation of excitation waves leaving field CA1 is facilitated after tetanization of Schaffer collaterals and during sleep,” Zh. Vyssh. Nerv. Deyat., 59, No. 1, 87–97 (2009).
C. A. Zosimovskii, V. A. Korshunov, and V. A. Markevich, “Conditions for the appearance of a double response to application of single stimuli to Schaffer collaterals in hippocampal field CA1 in freely moving rats,” Zh. Vyssh. Nerv. Deyat., 57, No. 2, 210–220 (2007).
C. W. Ang, G. C. Carlson, and D. A. Coulter, “Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies,” J. Neurosci., 25, No. 42, 9567–9580 (2005).
R. Bartesaghi and T. Gessi, “Activation of perforant path neurons to field CA1 by hippocampal projections,” Hippocampus, 13, No. 2,235–249 (2003).
R. Bartesaghi and T. Gessi, “Parallel activation of field CA2 and dentate gyrus by synaptically elicited perforant path volleys,” Hippocampus, 14, No. 8, 948–963 (2004).
R. Bartesaghi, M. Migliore, and T. Gessi, “Input-output relations in the entorhinal cortex-dentate-hippocampal system: evidence for a non-linear transfer of signals,” Neurosci., 142, No. 1, 247–265 (2006).
V. H. Brun, M. K. Otnass, S. Molden, H. A. Steffenach, M. P. Witter, M. B. Moser, and E. I. Moser, “Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry,” Science, 296, No. 5576, 2243–2246 (2002).
G. Buzsaki, “Two-stage model of memory trace formation: a role for ‘noisy’ brain states,” Neurosci., 31, No. 3, 551–570 (1989).
G. Buzsaki and E. Eidelberg, “Convergence of associational and commissural pathways on CA1 pyramidal cells of the rat hippocampus,” Brain Res., 237, No. 2, 283–295 (1982).
K. J. Canning, K. Wu, P. Peloquin, F. Kloosterman, and L. S. Leung, “Physiology of the entorhinal and perirhinal projections to the hippocampus studied by current source density analysis,” Ann. N.Y. Acad. Sci., 911, 55–72 (2000).
J. J. Chrobak and G. Buzsaki, “Selective activation of deep layer (V–VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat,” J. Neurosci., 14, No. 10, 6160–6170 (1994).
C. M. Colbert and W. B. Levy, “Electrophysiological and pharmacological characterization of perforant path synapses in CA1: mediation by glutamate receptors,” J. Neurophysiol., 68, 1–8 (1992).
R. M. Empson and U. Heinemann, “The perforant path projection to hippocampal area CA1 in the rat hippocampal-entorhinal cortex combined slice,” J. Physiol., 484, 707–720 (1995).
V. Gnatkovski and M. de Curtis, “Hippocampus-mediated activation of superficial and deep layer neurons in the medial entorhinal cortex of the isolated guinea pig brain,” J. Neurosci., 26, No. 3, 873–881 (2006).
O. Herreras, J. M. Solis, M. D. Munoz, R. Martin del Rio, and J. Lerma, “Sensory modulation of hippocampal transmission. I. Opposite effects on CA1 and dentate gyrus synapses,” Brain Res., 461, No. 2, 290–302 (1988).
R. Kajiwara, F. G. Wouterlood, A. Sah, A. J. Boekel, L. T. Baks-te Bulte, and M. P. Witter, “Convergence of entorhinal and CA3 inputs onto pyramidal neurons and interneurons in hippocampal area CA1 – an anatomical study in the rat,” Hippocampus, 18, No. 3, 266–280 (2008).
J. Kiss, G. Buzsaki, J. S. Morrow, S. B. Glantz, and C. Leranth, “Entorhinal cortical innervation of parvalbumin-containing neurons (Basket and Chandelier cells) in the rat Ammon’s horn,” Hippocampus, 6, No. 3, 239–246 (1996).
F. Kloosterman, T. Van Haeften, and F. H. Lopes da Silva, “Two reentrant pathways in the hippocampal-entorhinal system,” Hippocampus, 14, No. 8, 1026–1039 (2004).
P. Lavanex and D. G. Amaral, “Hippocampal-neocortical interaction: a hierarchy of associativity,” Hippocampus, 10, 420–430 (2000).
B. L. McNaughton, C. A. Barnes, J. Meltzer, and R. J. Sutherland, “Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge,” Exp. Brain Res., 76, No. 3, 485–496 (1989).
R. G. Morris, “Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas,” Eur. J. Neurosci., 23, No. 11, 2829–2846 (2006).
D. Pare, M. de Curtis, and R. Llinas, “Role of the hippocampalentorhinal loop in temporal lobe epilepsy: extra- and intracellular study in the isolated guinea pig brain in vitro,” J. Neurosci., 12, No. 5, 1867–1881 (1992).
M. Remondes and E. M. Schuman, “Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory,” Nature‚ 431, No. 7009, 699–703 (2004).
S. Riberiro and M. A. L. Nicolelis, “Reverberation, storage, and postsynaptic propagation of memories during sleep,” Learn. Mem., 11, No. 6, 686–696 (2004).
N. Tomamaki, K. Abe, and Y. Nojyo, “Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique,” Brain Res., 452, No. 1–2, 255–272 (1988).
I. Vida, K. Halasy, C. Szinyei, P. Somogyi, and E. H. Buhl, “Unitary IPSP evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro,” J. Physiol., 506, No. 3, 755–773 (1998).
N. A. Vorobyov and M. W. Brown, “The topography of activity transmission between lateral entorhinal cortex and subfield CA1 of the hippocampus,” Eur. J. Neurosci., 27, No. 12, 3257–3272 (2008).
H. Wang, Y. Hu, and J. Z. Tsien, “Molecular and systems mechanisms of memory consolidation and storage,” Progr. Neurobiol., 79, No. 3, 123–135 (2006).
M. P. Witter, F. G. Wouterlood, P. A. Naber, and T. Van Haeften, “Anatomical organization of the parahippocampal-hippocampal network,” Ann. N.Y. Acad. Sci., 911, 1–24 (2000).
T. Wolansky, E. A. Clement, S. R. Peters, M. A. Palczak, and C. T. Dickson, “Hippocampal slow oscillations: a novel EEG state and its coordination with ongoing neocortical activity,” J. Neurosci., 26, No. 23, 6213–6229 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 60, No. 5, pp. 568–581, September–October, 2010.
Rights and permissions
About this article
Cite this article
Zosimovskii, V.A., Korshunov, V.A. Excitation Waves Returning to the Hippocampus via the Entorhinal Cortex Can Reactivate Populations of “Trained” Field CA1 Neurons during Deep Sleep. Neurosci Behav Physi 42, 133–143 (2012). https://doi.org/10.1007/s11055-011-9546-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-011-9546-y