Abstract
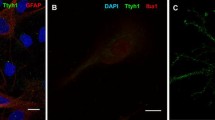
SC1 is an extracellular matrix molecule prominent in the mammalian brain. In the cerebellum, SC1 localizes to Bergmann glial cells and perisynaptic glial processes that envelop synapses in the molecular layer. In the present study, confocal microscopy revealed a punctate distribution of SC1 along Bergmann glial fibers that colocalized with the intermediate filament GFAP when fibers were viewed in cross-section. Immunoelectron microscopy showed that the punctate SC1 pattern corresponded to the localization of SC1 in multivesicular bodies situated within Bergmann glial fibers. The pattern of SC1 localization was not disrupted following hyperthermia or pilocarpine-induced status epilepticus. The present study suggests that SC1 protein may reach its destination in perisynaptic glial processes and glial endfeet by transport along Bergmann glial fibers in multivesicular bodies and that this process is preserved following stress.




Similar content being viewed by others
References
Novak U, Kaye AH (2000) Extracellular matrix and the brain: components and function. J Clin Neurosci 7:280–290
O’Shea KS, Dixit VM (1988) Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol 107:2737–2748
Rogers SL, Letourneau PC, Pech IV (1989) The role of fibronectin in neural development. Dev Neurosci 11:248–265
Venstrom KA, Reichardt LF (1990) Extracellular matrix. 2: role of extracellular matrix molecules and their receptors in the nervous system. FASEB J 7:996–1003
Johnston IG, Paladino T, Gurd JW et al (1990) Molecular cloning of SC1: a putative brain extracellular matrix glycoprotein showing partial similarity to osteonectin/BM40/SPARC. Neuron 4:165–176
Hennig A, Krueger R, Mangoura D et al (1992) Chondroitin sulfate proteoglycan expression during neuronal development. Cell Mol Biol 38:585–593
O’Connor LT, Lauterborn JC, Gall CM et al (1994) Localization and alternative splicing of agrin mRNA in adult rat brain: transcripts encoding isoforms that aggregate acetylcholine receptors are not restricted to cholinergic regions. J Neurosci 14:1141–1152
Seidenbecher CI, Richter K, Rauch U et al (1995) Brevican, a chondroitin sulfate proteoglycan of rat brain, occurs as secreted and cell surface glycosylphosphatidylinositol-anchored isoforms. J Biol Chem 270:27206–27212
Nikonenko I, Jourdain P, Muller D (2003) Presynaptic remodeling contributes to activity-dependent synaptogenesis. J Neurosci 23:8498–8505
Kiryushko D, Berezin V, Bock E (2004) Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci 1014:140–154
Ronn LC, Hartz BP, Bock E (1998) The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol 33:853–864
Garner CC, Zhai RG, Gundelfinger ED, Ziv NE (2002) Molecular mechanisms of CNS synaptogenesis. Trends Neurosci 25:243–251
Brekken RA, Sage EH (2001) SPARC, a matricellular protein: at the crossroads of cell–matrix communication. Matrix Biol 19:816–827
Sage EH (2001) Regulation of interactions between cells and extracellular matrix: a command performance on several stages. J Clin Invest 107:781–783
Martinek N, Zou R, Berg M et al (2002) Evolutionary conservation and association of SPARC with the basal lamina in Drosophila. Dev Genes Evol 212:124–133
Mendis DB, Shahin S, Gurd JW et al (1994) Developmental expression in the rat cerebellum of SC1, a putative brain extracellular matrix glycoprotein related to SPARC. Brain Res Mol Brain Res 633:197–205
Mendis DB, Shahin S, Gurd JW, Brown IR (1996) SC1, a SPARC-related glycoprotein, exhibits features of an ECM component in the developing and adult brain. Brain Res 713:53–63
Lively S, Brown IR (2007) Analysis of the extracellular matrix protein SC1 during reactive gliosis in the rat lithium-pilocarpine seizure model. Brain Res 1163:1–9
Yamada K, Watanabe M (2002) Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int 77:94–108
Bellamy TC (2006) Interactions between Purkinje neurones and Bergmann glia. Cerebellum 5:116–126
Lively S, Ringuette MJ, Brown IR (2007) Localization of the extracellular matrix protein SC1 to synapses in the adult rat brain. Neurochem Res 326:5–71
Lively S, Brown IR (2008) Localization of the extracellular matrix protein SC1 coincides with synaptogenesis during rat postnatal development. Neurochem Res 33:1692–1700
Mothe AJ, Brown IR (2002) Effect of hyperthermia on the transport of mRNA encoding the extracellular matrix glycoprotein SC1 into Bergmann glial cell processes. Brain Res 931:146–158
Belay HT, Brown IR (2003) Spatial analysis of cell death and Hsp70 induction in brain, thymus, and bone marrow of the hyperthermic rat. Cell Stress Chaperones 8:395–404
Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR (2005) Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J Neurosci Res 81:522–529
Chen S, Brown IR (2007) Translocation of constitutively expressed heat shock protein Hsc70 to synapse-enriched areas of the cerebral cortex after hyperthermic stress. J Neurosci Res 85:402–409
Glien M, Brandt C, Potschka H, Voigt H, Ebert U, Löscher W (2001) Repeated low-dose treatment of rats with pilocarpine: low mortality but high proportion of rats developing epilepsy. Epilepsy Res 46:111–119
Lively S, Brown IR (2008) The extracellular matrix protein SC1/hevin localizes to excitatory synapses following status epilepticus in the rat lithium-pilocarpine seizure model. J Neurosci Res 86:2895–2905
Lively S, Brown IR (2008) Extracellular matrix protein SC1/hevin in the hippocampus following pilocarpine-induced status epilepticus. J Neurochem 107:1335–1346
Racine RJ (1972) Modification of seizure activity by electrical stimulation, II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N (1997) KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron 18:425–438
Weible MW 2nd, Hendry IA (2004) What is the importance of multivesicular bodies in retrograde axonal transport in vivo? J Neurobiol 58:230–243
Herndon RM (1964) The fine structure of the rat cerebellum: II. The stellate neurons, granule cells, and glia. J Cell Biol 23:277–293
Castejon OJ (1990) Surface and membrane morphology of Bergmann glial cells and their topographic relationships in the cerebellar molecular layer. J Submicrosc Cytol Pathol 22:123–134
Theodosis DT, Poulain DA (1999) Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol 468:175–182
Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120:421–433
Kaether C, Skehel P, Dotti CG (2000) Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol Biol Cell 11:1213–1224
Grosche J, Kettenmann H, Reichenbach A (2002) Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res 68:138–149
Eng LF (1985) Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol 8:203–214
Trimmer PA, Phillips LL, Steward O (1991) Combination of in situ hybridization and immunocytochemistry to detect messenger RNAs in identified CNS neurons and glia in tissue culture. J Histochem Cytochem 39:891–898
Medrano S, Steward O (2001) Differential mRNA localization in astroglial cells in culture. J Comp Neurol 430:56–71
Müller M, Heuck A, Niessing D (2007) Directional mRNA transport in eukaryotes: lessons from yeast. Cell Mol Life Sci 64:171–180
Morimoto RI, Kline MP, Bimston DN, Cotto JJ (1997) The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem 32:17–29
Sonna LA, Fujita J, Gaffin SL, Lilly CM (2002) Invited review: effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92:1725–1742
Acknowledgments
We thank Professor James W. Gurd and Crystal Dykstra for the methodology of the rat lithium-pilocarpine seizure model. This work was supported by grants to I.B. from the National Science and Engineering Research Council of Canada who also holds a Canada Research Chair.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lively, S., Brown, I.R. The Extracellular Matrix Protein SC1/Hevin Localizes to Multivesicular Bodies in Bergmann Glial Fibers in the Adult Rat Cerebellum. Neurochem Res 35, 315–322 (2010). https://doi.org/10.1007/s11064-009-0057-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0057-y