Abstract
Objectives
To assess the potential of T1 mapping–based extracellular volume fraction (ECV) for the identification of higher grade clear cell renal cell carcinoma (cRCC), based on histopathology as the reference standard.
Methods
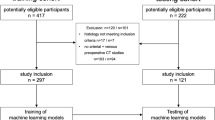
For this single-center, institutional review board–approved prospective study, 27 patients (17 men, median age 62 ± 12.4 years) with pathologic diagnosis of cRCC (nucleolar International Society of Urological Pathology (ISUP) grading) received abdominal MRI scans at 1.5 T using a modified Look-Locker inversion recovery (MOLLI) sequence between January 2017 and June 2018. Quantitative T1 values were measured at different time points (pre- and postcontrast agent administration) and quantification of the ECV was performed on MRI and histological sections (H&E staining).
Results
Reduction in T1 value after contrast agent administration and MR-derived ECV were reliable predictors for differentiating higher from lower grade cRCC. Postcontrast T1diff values (T1diff = T1 difference between the native and nephrogenic phase) and MR-derived ECV were significantly higher for higher grade cRCC (ISUP grades 3–4) compared with lower grade cRCC (ISUP grades 1–2) (p < 0.001). A cutoff value of 700 ms could distinguish higher grade from lower grade tumors with 100% (95% CI 0.69–1.00) sensitivity and 82% (95% CI 0.57–0.96) specificity. There was a positive and strong correlation between MR-derived ECV and histological ECV (p < 0.01, r = 0.88). Interobserver agreement for quantitative longitudinal relaxation times in the T1 maps was excellent.
Conclusions
T1 mapping with ECV measurement could represent a novel in vivo biomarker for the classification of cRCC regarding their nucleolar grade, providing incremental diagnostic value as a quantitative MR marker.
Key Points
• Reduction in MRI T1 relaxation times after contrast agent administration and MR-derived extracellular volume fraction are useful parameters for grading of clear cell renal cell carcinoma (cRCC).
• T1 differences between the native and the nephrogenic phase are higher for higher grade cRCC compared with lower grade cRCC and MRI-derived extracellular volume fraction (ECV) and histological ECV show a strong correlation.
• T1 mapping with ECV measurement may be helpful for the noninvasive assessment of cRCC pathology, being a safe and feasible method, and it has potential to optimize individualized treatment options, e.g., in the decision of active surveillance.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUA:
-
American Urological Association
- cRCC:
-
Clear cell renal cell carcinoma
- DWI:
-
Diffusion-weighted imaging
- ECM:
-
Extracellular matrix
- ECV:
-
Extracellular volume fraction
- ESM:
-
Electronic supplementary material
- H&E:
-
Hematoxylin and eosin
- ICC:
-
Intraclass coefficient
- ISUP:
-
International Society of Urological Pathology
- MOLLI:
-
Modified Look-Locker inversion recovery
- ROC:
-
Receiver operating characteristic curve
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
- TER:
-
Tumor enhancement ratio
References
Rini BI, Campbell SC, Escudier B (2009) Renal cell carcinoma. Lancet 373:1119–1132
Kovacs G, Akhtar M, Beckwith BJ et al (1997) The Heidelberg classification of renal cell tumours. J Pathol 183:131–133
Ng CS, Wood CG, Silverman PM, Tannir NM, Tamboli P, Sandler CM (2008) Renal cell carcinoma: diagnosis, staging, and surveillance. AJR Am J Roentgenol 191:1220–1232
Delahunt B, McKenney JK, Lohse CM et al (2013) A novel grading system for clear cell renal cell carcinoma incorporating tumor necrosis. Am J Surg Pathol 37:311–322
Leveridge MJ, Finelli A, Kachura JR et al (2011) Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 60:578–584
Jayson M, Sanders H (1998) Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51:203–205
Xi Y, Yuan Q, Zhang Y et al (2018) Statistical clustering of parametric maps from dynamic contrast enhanced MRI and an associated decision tree model for non-invasive tumour grading of T1b solid clear cell renal cell carcinoma. Eur Radiol 28:124–132
Nakamori S, Dohi K, Ishida M et al (2018) Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc Imaging 11:48–59
Ulyte A, Katsaros VK, Liouta E et al (2016) Prognostic value of preoperative dynamic contrast-enhanced MRI perfusion parameters for high-grade glioma patients. Neuroradiology 58:1197–1208
Xu F, Chang K, Ma J et al (2017) The oncogenic role of COL23A1 in clear cell renal cell carcinoma. Sci Rep 7:9846
Lohi J, Leivo I, Oivula J, Lehto VP, Virtanen I (1998) Extracellular matrix in renal cell carcinomas. Histol Histopathol 13:785–796
Arheden H, Saeed M, Higgins CB et al (2000) Reperfused rat myocardium subjected to various durations of ischemia: estimation of the distribution volume of contrast material with echo-planar MR imaging. Radiology 215:520–528
Coy H, Young JR, Douek ML et al (2018) Association of qualitative and quantitative imaging features on multiphasic multidetector CT with tumor grade in clear cell renal cell carcinoma. Abdom Radiol (NY). https://doi.org/10.1007/s00261-018-1688-8
Ficarra V, Novara G, Galfano A et al (2009) The ‘Stage, Size, Grade and Necrosis’ score is more accurate than the University of California Los Angeles Integrated Staging System for predicting cancer-specific survival in patients with clear cell renal cell carcinoma. BJU Int 103:165–170
Campbell S, Uzzo RG, Allaf ME et al (2017) Renal mass and localized renal cancer: AUA Guideline. J Urol 198:520–529
Ljungberg B, Bensalah K, Canfield S et al (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67:913–924
Karlo CA, Donati OF, Burger IA et al (2013) MR imaging of renal cortical tumours: qualitative and quantitative chemical shift imaging parameters. Eur Radiol 23:1738–1744
Yoshimitsu K, Honda H, Kuroiwa T et al (1999) MR detection of cytoplasmic fat in clear cell renal cell carcinoma utilizing chemical shift gradient-echo imaging. J Magn Reson Imaging 9:579–585
Baliyan V, Das CJ, Sharma R, Gupta AK (2016) Diffusion weighted imaging: technique and applications. World J Radiol 8:785–798
Bickel H, Pinker-Domenig K, Bogner W et al (2015) Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 50:95–100
Rosenkrantz AB, Niver BE, Fitzgerald EF, Babb JS, Chandarana H, Melamed J (2010) Utility of the apparent diffusion coefficient for distinguishing clear cell renal cell carcinoma of low and high nuclear grade. AJR Am J Roentgenol 195:W344–W351
Lassel EA, Rao R, Schwenke C, Schoenberg SO, Michaely HJ (2014) Diffusion-weighted imaging of focal renal lesions: a meta-analysis. Eur Radiol 24:241–249
Hotker AM, Mazaheri Y, Wibmer A et al (2016) Use of DWI in the differentiation of renal cortical tumors. AJR Am J Roentgenol 206:100–105
Lopes Vendrami C, Parada Villavicencio C, DeJulio TJ et al (2017) Differentiation of solid renal tumors with multiparametric MR imaging. Radiographics 37:2026–2042
Kang SK, Zhang A, Pandharipande PV, Chandarana H, Braithwaite RS, Littenberg B (2015) DWI for renal mass characterization: systematic review and meta-analysis of diagnostic test performance. AJR Am J Roentgenol 205:317–324
Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2017) Diagnostic performance of DWI for differentiating high- from low-grade clear cell renal cell carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol 209:W374–W381
Hu G, Liang W, Wu M et al (2018) Comparison of T1 mapping and T1rho values with conventional diffusion-weighted imaging to assess fibrosis in a rat model of unilateral ureteral obstruction. Acad Radiol. https://doi.org/10.1016/j.acra.2018.03.023
Friedli I, Crowe LA, Berchtold L et al (2016) New magnetic resonance imaging index for renal fibrosis assessment: a comparison between diffusion-weighted imaging and T1 mapping with histological validation. Sci Rep 6:30088
Robbers LF, Baars EN, Brouwer WP et al (2012) T1 mapping shows increased extracellular matrix size in the myocardium due to amyloid depositions. Circ Cardiovasc Imaging 5:423–426
Iles L, Pfluger H, Phrommintikul A et al (2008) Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 52:1574–1580
Klatt MG, Kowalewski DJ, Schuster H et al (2016) Carcinogenesis of renal cell carcinoma reflected in HLA ligands: a novel approach for synergistic peptide vaccination design. Oncoimmunology 5:e1204504
Ho TH, Serie DJ, Parasramka M et al (2017) Differential gene expression profiling of matched primary renal cell carcinoma and metastases reveals upregulation of extracellular matrix genes. Ann Oncol 28:604–610
Wan F, Wang H, Shen Y et al (2015) Upregulation of COL6A1 is predictive of poor prognosis in clear cell renal cell carcinoma patients. Oncotarget 6:27378–27387
Paudyal B, Paudyal P, Tsushima Y et al (2010) The role of the ADC value in the characterisation of renal carcinoma by diffusion-weighted MRI. Br J Radiol 83:336–343
White SK, Sado DM, Fontana M et al (2013) T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging 6:955–962
Acknowledgements
LCA and BR are grateful for their participation in the BIH Charité - Junior Clinician Scientist Program funded by the Charité - Universitaetsmedizin Berlin and the Berlin Institute of Health. JB is participant in the BIH – Twinning Grant Program funded by the Charité - Universitaetsmedizin Berlin and the Berlin Institute of Health. MRM is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB 1340/1 2018, 5943/31/41.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Lisa C. Adams.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Bernd Hamm has received research grants for the Department of Radiology, Charité – Universitätsmedizin Berlin from the following companies: 1. Abbott, 2. Actelion Pharmaceuticals, 3. Bayer Schering Pharma, 4. Bayer Vital, 5. BRACCO Group, 6. Bristol-Myers Squibb, 7. Charite research organisation GmbH, 8. Deutsche Krebshilfe, 9. Dt. Stiftung für Herzforschung, 10. Essex Pharma, 11. EU Programmes, 12. Fibrex Medical Inc., 13. Focused Ultrasound Surgery Foundation, 14. Fraunhofer Gesellschaft, 15. Guerbet, 16. INC Research, 17. lnSightec Ud., 18. IPSEN Pharma, 19. Kendlel MorphoSys AG, 20. Lilly GmbH, 21. Lundbeck GmbH, 22. MeVis Medical Solutions AG, 23. Nexus Oncology, 24. Novartis, 25. Parexel Clinical Research Organisation Service, 26. Perceptive, 27. Pfizer GmbH, 28. Philipps, 29. Sanofis-Aventis S.A, 30. Siemens, 31. Spectranetics GmbH, 32. Terumo Medical Corporation, 33. TNS Healthcare GMbH, 34. Toshiba, 35. UCB Pharma, 36. Wyeth Pharma, 37. Zukunftsfond Berlin (TSB), 38. Amgen, 39. AO Foundation, 40. BARD, 41. BBraun, 42. Boehring Ingelheimer, 43. Brainsgate, 44. PPD (Clinical Research Organisation), 45. CELLACT Pharma, 46. Celgene, 47. CeloNova BioSciences, 48. Covance, 49. DC Deviees, Ine. USA, 50. Ganymed, 51. Gilead Sciences, 52. Glaxo Smith Kline, 53. ICON (Clinical Research Organisation), 54. Jansen, 55. LUX Bioseienees, 56. MedPass, 57. Merek, 58. Mologen, 59. Nuvisan, 60. Pluristem, 61. Quintiles, 62. Roehe, 63. Sehumaeher GmbH (Sponsoring eines Workshops), 64. Seattle Geneties, 65. Symphogen, 66. TauRx Therapeuties Ud., 67. Accovion, 68. AIO: Arbeitsgemeinschaft Internistische Onkologie, 69. ASR Advanced sleep research, 70. Astellas, 71. Theradex, 72. Galena Biopharma, 73. Chiltern, 74. PRAint, 75. lnspiremd, 76. Medronic, 77. Respicardia, 78. Silena Therapeutics, 79. Spectrum Pharmaceuticals, 80. St. Jude., 81. TEVA, 82. Theorem, 83. Abbvie, 84. Aesculap, 85. Biotronik, 86. Inventivhealth, 87. ISA Therapeutics, 88. LYSARC, 89. MSD, 90. novocure, 91. Ockham oncology, 92. Premier-research, 93. Psi-cro, 94. Tetec-ag, 94. Tetec-ag, 95. Winicker-norimed, 96. Achaogen Inc, 97. ADIR, 98. AstraZenaca AB, 99. Demira Inc, 100. Euroscreen S.A., 101. Galmed Research and Development Ltd., 102. GETNE, 103. Guidant Europe NV, 104. Holaira Inc., 105. Immunomedics Inc., 106. Innate Pharma, 107. Isis Pharmaceuticals Inc, 108. Kantar Health GmbH, 109. MedImmune Inc, 110. Medpace Germany GmbH (CRO), 111. Merrimack Pharmaceuticals Inc, 112. Millenium Pharmaceuticals Inc, 113. Orion Corporation Orion Pharma, 114. Pharmacyclics Inc, 115. PIQUR Therapeutics Ltd, 116. Pulmonx International Sárl, 117. Servier (CRO), 118. SGS Life Science Services (CRO), 119. Treshold Pharmaceuticals Inc. There are no patents, products in development or marketed products to declare. This does not alter the authors’ adherence to all the European Radiology policies on sharing data and materials.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study (proof-of-concept study)
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1280 kb)
Rights and permissions
About this article
Cite this article
Adams, L.C., Jurmeister, P., Ralla, B. et al. Assessment of the extracellular volume fraction for the grading of clear cell renal cell carcinoma: first results and histopathological findings. Eur Radiol 29, 5832–5843 (2019). https://doi.org/10.1007/s00330-019-06087-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06087-x