Abstract
Many populations of European migrant bird species are declining and this may be driven by survival rates; however, there are few studies that can estimate true survival rates. Cyprus wheatears Oenanthe cypriaca are an endemic migrant that winter in East Africa: populations are probably not declining but are annually variable. We recorded territory occupation and reoccupation in a colour-ringed population of 45–69 pairs over a 4-year period (2010–2013) from April to August to measure apparent survival and determine how it varied with sex, age, breeding productivity and year. We then estimated true survival by correcting apparent survival for dispersal by recording territory shifts and how this also varied by sex, age, breeding productivity and year. Apparent annual survival rate varied significantly by sex, age and year (males 2011, 2012, 2013: 0.70, 0.50, 0.62; females: 0.56, 0.34, 0.47; chicks: 0.35, 0.19, 0.28) but was not affected by the productivity of a territory. An average of 1.1 % of males and 8.2 % of females were lost during breeding, where 5/7 lost females were found depredated during incubation. Adults did not usually change territories between years (87 % were resident and 99 % moved less than four territories between years) regardless of sex, productivity or year; chicks, independent of their sex, moved on average three territories away from their natal territory. After correcting apparent survival for the probability of dispersal, males had the highest true minimum annual survival compared to females which were very similar to chicks (males 2011, 2012, 2013: 0.77, 0.50, 0.65; females: 0.65, 0.35, 0.50; chicks: 0.64, 0.34, 0.49). The results indicate a very high survival rate for a small passerine migrant, although they are probably sufficiently annually variable to profoundly affect annual population dynamics. If females have a lower survival rate, the sex ratio at birth may be female-biased to compensate; alternatively, females may have longer range dispersal than we could measure, particularly if they respond to their mates not returning by moving territories, leading to underestimation of their true survival. A high survival rate may be due to the rarity of sparrowhawks on Cyprus and the wheatears relatively short distance migration.
Zusammenfassung
Überleben und Ausbreitung des Zypernsteinschmätzers ( Oenanthe cypriaca ), einem endemischen Zugvogel
Viele Populationen europäischer Zugvögel sind im Rückgang begriffen. Dies könnte auf einen Rückgang der Überlebensraten zurückzuführen sein, doch gibt es nur wenige Studien, welche die tatsächlichen Überlebensraten abschätzen können. Der Zypernsteinschmätzer (Oenanthe cypriaca) ist ein endemischer Zugvogel, der in Ostafrika überwintert; seine Populationen sind wahrscheinlich nicht rückläufig, sondern im Jahresverlauf variabel. Wir haben das scheinbare Überleben von Zypernsteinschmätzern gemessen, indem wir die Besetzung und Wiederbesetzung von Revieren in einer farbberingten Population mit 45–69 Paaren über vier Jahre (2010–2013) von April bis August erfasst haben und festgestellt haben, ob diesbezüglich Geschlechts-, Alters- und Jahresunterschiede bestehen. Wir haben dann das tatsächliche Überleben abgeschätzt, indem wir Revierwechsel erfasst und das scheinbare Überleben mittels dieser Ausbreitungsdaten korrigiert haben; hierbei haben wir ebenfalls Geschlechts-, Alters- und Jahresunterschiede berücksichtigt. Die scheinbare jährliche Überlebensrate variierte signifikant zwischen den Geschlechtern, Alt- und Jungvögeln und den verschiedenen Jahren (Männchen 2011, 2012, 2013: 0,70, 0,50, 0,62; Weibchen: 0,56, 0,34, 0,47; Jungvögel: 0,35, 0,19, 0,28), wurde jedoch nicht von der Produktivität des Reviers beeinflusst. Durchschnittlich 1,1 % der Männchen und 8,2 % der Weibchen, die mit der Brut begonnen hatten, konnten später nicht mehr erfasst werden, wobei fünf von sieben Weibchen während der Bebrütungsphase von Räubern erbeutet wurden. Unabhängig von Geschlecht, Produktivität oder Jahr wechselten Altvögel ihr Revier zwischen den Jahren normalerweise nicht (87 % blieben im selben Revier und 99 % siedelten sich in einem Revier an, das weniger als vier Reviere von ihrem vorherigen Revier entfernt war); Jungvögel besetzten unabhängig von ihrem Geschlecht ein Revier, das durchschnittlich drei Reviere von ihrem Geburtsrevier entfernt lag. Nachdem das scheinbare Überleben mittels der Ausbreitungswahrscheinlichkeit korrigiert worden war, wiesen Männchen das höchste tatsächliche Mindestüberleben von einem Jahr zum nächsten auf, während das Überleben der Weibchen stark dem der Jungvögel ähnelte (Männchen 2011, 2012, 2013: 0,77, 0,50, 0,65; Weibchen: 0,65, 0,35, 0,50; Jungvögel: 0,64, 0,34, 0,49). Diese Ergebnisse zeigen eine für einen kleinen ziehenden Sperlingsvogel sehr hohe Überlebensrate, wobei die Überlebensraten wahrscheinlich hinreichend variabel sind, um die jährliche Populationsdynamik deutlich zu beeinflussen. Falls die Weibchen tatsächlich geringere Überlebensraten haben, könnte dies durch eine Verschiebung des Geschlechterverhältnisses der Jungvögel zu Weibchen hin ausgeglichen werden. Alternativ könnten Weibchen sich über längere Strecken als die von uns gemessenen ausbreiten, besonders falls sie ihr Revier wechseln, wenn ihr Partner nicht zurückkehrt (was zu einer Unterschätzung ihres tatsächlichen Überlebens führen würde). Das allgemein hohe Überleben könnte darauf zurückzuführen sein, dass Sperber auf Zypern selten sind und die Steinschmätzer nur über relativ kurze Strecken ziehen.
Similar content being viewed by others
Introduction
Many populations of European migrant bird species are declining, and measuring survival rates at the different stages in their annual cycle is crucial to understand why this is so (Newton 2004, 2008). Studies on migrant bird species that breed in Europe and winter in sub-Saharan Africa have showed that their rate of decline is greater than residents and short-distance migrants (Sanderson et al. 2006; Jones and Cresswell 2010). Populations of Afro–Palearctic migrants declined over large parts of Europe between 1970 and 2000, particularly for species wintering in arid open habitats in Africa (Sanderson et al. 2006); more recent analyses have identified that species wintering in the Guinea savannah may now have greater declines (Thaxter et al. 2010; Ockendon et al. 2012). The causes of these declines are not well known and are likely to be species-specific; for example, reduced rainfall and, as a result, habitat quality in the wintering grounds (Kanyamibwa et al. 1993), habitat quality of stop-over sites (Saino et al. 2004; Schaub et al. 2005), or phenology mismatch (Both and te Marvelde 2007; Jones and Cresswell 2010).
Survival rate is an important fitness component, and accurate measurement of this at different life history stages is, therefore, essential to understand and manage any declining population. Environmental conditions such as weather and food availability (Peach et al. 1999; Robinson et al. 2007), as well as density-dependent processes (Saether and Engen 2002), are the important factors shaping survival. Survival may, however, also be related to age (Clobert et al. 1988), sex (Post and Gotmark 2006), or may vary between individuals according to their social status (Schubert et al. 2008). Reproductive efforts can also impact adult survival rates (McCleery et al. 1996).
Population declines arise because productivity is less than annual mortality (Newton 1998). Population dynamics may, therefore, be driven by survival rates of adults and, particularly, during the first years (Arlt et al. 2008). The greatest mortality of migrants may occur when they migrate (Sillett and Holmes 2002), although events and conditions during the breeding season, such as variation in food availability (Arlt and Part 2008), may also affect the cost of reproduction and, consequently, survival of adult birds due to the increase of parental workload when food is scarce (Low et al. 2010). There may be mortality specifically associated with breeding and its constraints, such as restriction to a fixed site during incubation and chick rearing, and where one sex has a greater role in this, sex-specific mortality may arise. During the breeding cycle, males searching and competing for mates and territories usually have high levels of mobility, signalling and display behaviours, which may entail higher risk of exposure of males to predators (Jakobsson et al. 1995; Zuk and Kolluru 1998). Females need to forage extensively to meet energy requirements during egg production and incubation, which may make them less vigilant (Dukas and Kamil 2000) and may significantly increase their mass when forming eggs, which may impair their flight performance and ability to escape predators (Witter et al. 1994). Predation may also be higher during incubation for females that are killed in the nest by mammalian nest predators (Brasher et al. 2006; Krams et al. 2014). Consequently, if females are the sole incubators, they may have an enhanced risk of predation during incubation (Grubler et al. 2008; Perlut et al. 2008).
The first year of life may also represent a time of particularly high mortality. Juvenile survival has been universally found to be lower than adult survival in migrants (Saether 1989; Saether and Bakke 2000; Donovan et al. 1995) and this disparity arises because juveniles initially have no experience with their migration routes or wintering grounds, and also because of their inexperience in foraging ability (Desrochers 1992) or habitat choice (Cresswell 1994). The first migration, as compared to subsequent residency periods, in any migrant species results in relatively lower survival because of its associated uncertainties that are substantially reduced when adults gain experience with a survivable migration route and wintering area (Strandberg et al. 2010). As a result, juveniles that have established territories in the wintering grounds tend to have the same survival rate as adults (Sillett and Holmes 2002).
Although knowledge of survival rates are crucial for understanding population dynamics, they are rarely measured accurately. Estimating true survival rates for adults and juvenile migrants is difficult because estimates are confounded by site fidelity (apparent survival): birds that have not returned to a study area may have died or may have dispersed (e.g. Doligez and Part 2008). This is a particular problem for estimating juvenile survival because all juveniles tend to disperse away from their natal site to some degree during their first year (e.g. Gardali et al. 2003). True survival estimates require dispersal to be accounted for and, so, require large-scale studies or the estimate must be for species that have only small-scale dispersal. For example, in studies of adult survival rates of the Northern wheatear (Oenanthe oenanthe), breeding in Sweden probably reflected true survival (Low et al. 2010), because the studies minimised the effect of breeding dispersal by surveying within a radius of 2–4 km from the central study area between consequent years.
Here, we measure survival in a Palearctic migrant, the Cyprus wheatear. Cyprus wheatears are widespread and common migrants endemic to Cyprus that winter in sub-Saharan East Africa from southern Sudan to Ethiopia (N = 6 geolocator birds from this study 2014–2015, unpublished data). They have a broad niche range, breeding from sea level up to the highest mountains in Cyprus, inhabiting diverse habitats types, including open, stony areas, urban areas, as well as shrub, scrub, and bush vegetation and sparse coniferous woodland (Flint and Stewart 1992; Randler et al. 2010). Populations of Cyprus wheatears are probably not declining but are probably annually variable. The population size of Cyprus wheatears is approximately 90,000–180,000 breeding pairs and its population status is regarded as secure (Randler et al. 2010). The population of Cyprus wheatears may be stable in the long term, but there are likely to be annual fluctuations in populations due to varying productivity and survival as a consequence of annual environmental and anthropogenic variation.
We recorded territory occupation and reoccupation in a colour-ringed population over a 4-year period (2010–2013) from April to August to measure apparent survival and determine how it varied with sex, age, breeding productivity and year. We then estimated true survival by correcting apparent survival for dispersal by recording territory shifts and how this also varied by sex, age, breeding productivity and year.
Methods
The study was conducted from 2009 to 2013 in a 130-ha area at Troodos National Forest Park (34°56′11″N 32°51′48″E), at about 1800 m a.s.l. on the Island of Cyprus during the breeding season. The study area was surrounded by the “Artemis Trail” with an old, low-density coniferous forest, supporting one of the densest breeding Cyprus wheatear populations on the island (Flint and Stewart 1992). The National Forest Park of Troodos (NFP of Troodos) is located at the centre of the Troodos massif that ranges from the northwest to the southeast part of Cyprus. The NFP of Troodos covers an area of 9029 ha with its highest peak of Chionistra at 1952 m, situated almost at the centre of an ophiolite complex. The main habitats that characterized the study area are those of the endemic Pinus nigra ssp. pallasiana forest, the black pine zone which starts from 1400 m and extends to the top of the mountains at 1952 m, and Juniperus foetidissima woodland and Serpentinophilous grasslands that are distributed at the highest parts and that occur in openings of the black pine forest in the form of small, scattered patches. The understorey of Black pine consists of Quercus alnifolia, Juniperus oxycedrus, J. foetidissima, Sorbus aria ssp. cretica, Berberis cretica, Arbutus andrachne, Rosa chionistrae, Rosa canina, Cotoneaster racemiflorus var. nummularius, etc. Annual precipitation in the area is very high with more than 1100 mm being recorded, and temperature varies through the year from freezing during winter to a maximum of about 35 °C during very hot, dry summers.
During 2009, a pilot study identified a study area and monitored and mapped territories but without colour-ringing of birds. In 2010–2012, individuals were colour-ringed when they actively defended a territory, and locations of territories were plotted on maps; territories were intensively monitored to record details of breeding. During April–May 2013, only territory mapping and resighting was carried out, but in an expanded study area, covering a buffer zone of around 300 m around the previous study area. The 4-year study was conducted in an area consisting of 4 parallel transects containing about 70 mapped territories. Pairs were marked within their territory and at least one individual per pair was coloured-ringed. All territories were regularly monitored throughout the breeding season (at least every second/third day) and detailed data were collected from a central and intensively studied 130-ha part of the total study area, with the surrounding area monitored for adult and chick dispersal only during 2013. Note that survival rates for any quoted year refer to survival during the previous 12 months; thus, survival rates for 2012 are from the period May 2011 until the end of April 2012.
Observation and data recording started around the mid-end of March each year when the first individuals arrived at the breeding grounds, and regular monitoring continued until the end of August, with sporadic visits until October. Birds were captured with spring traps baited with maggots throughout the season (but especially during arrival and territory establishment for the adults) and ringed with individual combinations of colour rings (with permission from the Game Fund, Ministry of Interior and BirdLife Cyprus): playback using conspecific songs was used during trapping. Individuals were sexed, and all males were aged as young (1 year old) or adults (>1 year old) based on their plumage characteristics (contrast in the coverts so that first year males had brownish greater coverts—visible in the field—whereas, adult males had dark, uniformly sooty, black wing feathers). The age of the female was not considered to have been reliably distinguished in the first and second year due to inexperience and, so, female age is not considered here. Territories were mapped on paper using landmarks and via a Garmin 62 GPS unit. Territories were delimited by the outermost positions of the majority of all recorded positions of the resident pair or/and unpaired male. A GPS point was taken on the approximate centre of the territory sites (location), determined by multiple observations of colour-ringed birds (pairs) over several months, and nest location. Territory sites were largely stable across the years irrespective of territory holders. At each territory during 2010–2012, we recorded arrival dates of individuals, resighted colour-ringed birds, mapped territories, and monitored nesting stage to determine if any nest failures were associated with depredation of incubating females. The study contained 324 individuals that were colour ringed over 2010–2013 (110 males, 91 females and 123 chicks); 45 territories were monitored during 2010, 69 during 2011 and 50 during 2012.
We used resighting rates of individually marked Cyprus wheatears to determine the annual survival rate of adult migratory birds and their fledged offspring from 1 month after fledging. Cyprus wheatears are highly detectable. Typically, both male and female birds could be seen on every visit to a territory, particularly before incubation commenced. Cyprus wheatears, typically, will allow approach to within 30 m and their flushing response is to fly up to conspicuous perches rather than slipping away. They utilise lightly wooded habitats with large P. nigra trees that have a dense crown but few lower branches. The bare rocky hillsides of the study were largely devoid of low vegetation apart from occasional B. cretica and, in any case, wheatears perch conspicuously on any tall vegetation present in their territory. Males sing for long periods from conspicuous song perches. Both male and female birds respond to playback of song particularly in April before incubation commences (see also Randler et al. 2012), moving to within a few meters of a playback source within minutes. Males from adjacent territories and particularly males and females from territories that are establishing at the beginning of the season will also travel several 100 m to check out a playback source. Females make a soft chacking call when people are near their nests, adding to their detectability. Therefore, and also because of the intensive nature of the study, we considered a bird to have survived the previous winter if it was recorded at the study site the following April–May. No birds were ever not recorded in a season and then recorded in the next unless they had moved out of the study area (i.e. in 2013, when the search area was expanded to measure dispersal on a larger scale—see below). We considered a bird to have survived the breeding season if it was seen during August in its territory. Sample sizes of birds ringed and resighted in the different periods are given in Table 1.
Dispersal was estimated by observing movements between breeding territories for adults between years and between natal and their first breeding territories for chicks. Each year, all territories and territory holders were mapped and the number of territories moved for each bird was scored between consecutive years. Territories were close together: mean distance to the nearest territory from a focal territory (mapped approximately centre to centre to the nearest 10 m) was 88.9 ± 1.7 m (n = 164) across all years. In 2013, the study area was expanded to a distance of 2–3 territories (i.e. c. 300 m) beyond the study area used previously to increase sample sizes and to look for evidence that birds were surviving but being missed because of dispersal out of the study area. Six extra chicks from the previous year, one from 2 years previously, and one adult were found. These extra birds were not included in estimates of survival rates so that the 3-year rates were directly comparable, but were included in the dispersal distance analysis. All adults were ringed wherever they could be caught, but only a sample of chick broods were ringed each year, concentrating on chick broods within the centre of the study area to maximise the chance of resighting the following year even with dispersal.
The proportion of birds that we would have detected, assuming 100 % return but with average territory movements as recorded above, was estimated in the following way. The territory map for 2012 was used (the spatial arrangement and density of territories was similar in all years) and each territory was scored for the number of territories adjacent to it, 1 territory away, 2 territories away, and so on until 7 territories (the largest dispersal distance observed, c. 270 m) were in the study area (and, therefore, where we assumed a returning bird would be resighted), on each of the 4 ordinal compass bearings. Thus, a territory on the edge of the study area might have most of its adjacent territories—even at only one territory away—where a returning bird would not be resighted. In contrast, a central territory would have a very high proportion of its adjacent territories within the study area. Then, the proportion of adjacent territories in each distance band (1–7 territories away) within the study area was averaged across all territories (N = 50 territories): for example, 89 % of territories within 1 territory of the study area were monitored, 73 % within 2, 58 % within 3 and so on until 4 % within 7 territories. The proportion of birds that were observed moving one territory was then multiplied with the proportion of territories that were monitored to calculate the proportion of birds that would have been resighted. This was repeated for all the distance bands and then the proportions were summed to give the overall proportion of dispersing birds that would have been detected in the study. The calculations were carried out separately for chicks and adults because of their different recorded dispersal distances (see below).
Resighting data was then corrected for the probability of dispersal by dividing the total number of either adults or chicks resighted in each year with the estimated resighting rate for adults or chicks due to dispersal, respectively, to give the total number of birds in each age class that were likely not to have been recorded due to dispersal. This was a trivial number for adults in each year (0–1) but a larger number of chicks (6–8): see the “Results” section. Randomly chosen adults and chicks that were not resighted were then scored as having been resighted to match these annual estimated numbers of dispersing birds.
Analysis was carried out using R 2.13.1 (R Development Core Team 2014). Probability of survival was tested using resighting data for apparent survival and resighting data corrected for dispersal for true survival. Models to predict probability of resighting (0 or 1) were binomial logistic regressions with log–link functions. Overwinter survival considered all birds ringed that were seen alive at the end of the breeding season (August) that were then resighted the following April–May, and considered all chicks ringed. Chicks were all ringed in August apart from 4 in the last week of July and 11 in the first week of September (total N = 123). Annual survival considered all adult birds that were ringed between April and May and resighting during April–May the following year. Almost all adults were ringed in April and May apart from 3 in July and 14 in the first week of June (N = 201): these adults ringed April–May were all known individuals associated with successful breeding territories monitored during April–May. Models included, where necessary, individual identity of the bird (bird id) and brood identity (terr id) as random effects in the model because some adults survived more than 1 year and more than one chick from the same brood survived, respectively.
Models for overwinter survival considered were: probability of resighting (0/1) ~ age sex class + year + productivity + (1|bird id) + (1|terr id) + age sex class × year.
Note that there was no productivity associated with chicks, so the initial model only included adults. Productivity was not a significant predictor and neither was the interaction, so these variables were removed (see below).
Models for annual survival considered were: probability of resighting (0/1) ~ sex + year + productivity + (1|bird id) + sex × year.
Note that the annual models only included adults (because all chicks were ringed more or less in August). Productivity was not a significant predictor and neither was the interaction, so these variables were removed (see below).
Note also that a mark–recapture analysis was not well suited for our data, mainly because resighting and capture effort varied substantially between territories in a year and across the three study years. Only 2 (out of 201) adults were resighted with a gap between years (i.e. 101 in an annual mark–recapture analysis), but this was because these birds were captured on the boundary of the study sites and their territories were only located upon expanding the study boundaries in 2013, not because they went undetected within the study site. There were, therefore, no instances where a bird resident in the study area was not detected in 1 year but then detected the following year. Consequently, we used our simpler approach for establishing within-winter and annual survival.
Probability of dispersal was tested using territory movement data. Models to predict probability of dispersal (0 = stayed in territory, or 1 = changed territories between years) were binomial logistic regressions with log–link functions. Models for dispersal considered were: probability of moving (0/1) ~ sex + year + productivity + (1|bird id) + sex × year + sex × productivity.
Results
Both annual and overwinter (September–April inclusive) apparent survival rate varied significantly by sex, age and year but were not affected by the productivity of a territory (Table 2; Fig. 1). Males had the highest annual survival (0.50–0.70) compared to females (0.34–0.56) compared to chicks (0.19–0.35), and annual survival within an age or sex class varied by a maximum of about 21 %. Only 1.1 % of ringed males were lost during the breeding season (May–August inclusive) compared to 8.2 % of females; 71 % of females that were lost (N = 7) were found depredated during incubation (Table 1). Apparent overwinter survival rates were, therefore, very similar to annual apparent survival rates (Table 1; Fig. 1).
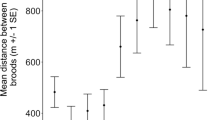
Predicted apparent overwinter survival (W; September–April inclusive) and annual survival (A) for Cyprus wheatears by year, age and sex. Mean parameter estimates and one standard error plotted are from the model in Table 2. Note that only one chick value is plotted per year because only overwinter survival rates are relevant (most chicks were fledged in August). The dashed line is a survival rate of 0.5, plotted to allow the values to be read more easily
The probability that a Cyprus wheatear changed territory between years was not dependent on sex, productivity or year (87 % of adult birds did not change territory, 12 % moved 1–4 territories away, and 1 % moved 6 territories away, N = 109: Table 3; Fig. 2). If an adult changed territories, the distance it moved did not depend on sex (t 1,10 = −0.9, P = 0.41) or on productivity (t 1,10 = 1.7, P = 0.12) or on the interaction between sex × productivity (t 1,10 = −1.0, P = 0.33).
Frequency distributions of the number of territories moved by adult males and females, and chicks in the following year (0 if they reoccupied the territory used in/their natal territory in the following year). The distributions of male and females are very similar (Kruskall–Wallis one-way analysis of variance [ANOVA] χ 2 = 0.1, P = 0.73)
More male chicks were resighted in a subsequent year compared to females (male n = 17, female n = 7: \(\chi_{1}^{2}\) = 4.4, P = 0.041). The distance moved by a chick was not, however, dependent on the sex of the chick (t 1,22 = −0.8, P = 0.46, including brood as a random effect). Chicks were significantly much more likely to change territories in their first year (mode 3 territories, range 0–7, N = 36) than adults (z = −6.1, P < 0.001, N = 139 including individual identity of adult birds and brood identity of chick as random effects: Fig. 3).
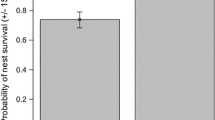
The probability of a chick or adult that survived over the winter moving from their natal or breeding territory, respectively, in the following breeding season. Mean parameter estimates and one standard error plotted are from the model in Table 3
The probability that an adult that survived the winter would return to a territory in the study site (assuming a dispersal pattern according to the observed distribution illustrated in Fig. 2) was calculated as 0.963 (i.e. Very few dispersed and, if they did, they moved only one or two territories; so, only some of those birds on the edge of the study area would be missed the next year). The probability that a chick that survived the winter would return to a territory in the study site (assuming a dispersal pattern according to the observed distribution illustrated in Fig. 2) was calculated as 0.650 (e.g. 81 % of chicks moved 3 territories or less, and 87 % of territories were within 3 territories of where chicks were ringed). This meant that we probably missed resighting six chicks and one male in 2010, eight chicks, one male and one female in 2011 and seven chicks, one male and one female in 2012. Consequently, corrected predicted minimum true survival rates were very similar to apparent survival rates for males and females, but were much higher for chicks (Table 4; Fig. 4) where their high dispersal rates led to substantial underestimates.
Predicted true overwinter survival (W September–April inclusive) and annual survival (A) for Cyprus wheatears by year, age and sex. Mean parameter estimates and one standard error plotted are from the model in Table 4. Note that only one chick value is plotted per year because only overwinter survival rates are relevant (some chicks were fledged in August). Mean number of resightings was corrected using an estimated probability of remaining in the study area of 0.963 for adults and 0.650 for chicks, if a bird survived. The dashed line is a survival rate of 0.5, plotted to allow the values to be read more easily
Males had the highest true minimum annual survival (0.50–0.77) compared to females (0.35–0.65) which were very similar to chicks (0.34–0.64). Note that the minimum actual overwinter survival rate for chicks in 2012–2013 (including the chicks found from the wider dispersal search) was 19/47 = 40.4 %: minimum real survival rates, without any correction, are, therefore, high (Table 1) with a mean for males of 60.0 %, females of 45.4 % and chicks of 31.4 %.
Discussion
Our study showed that Cyprus wheatears were highly site-faithful with little dispersal for adults and dispersal on the scale of only a few 100 m for first-year birds. Minimum estimates of true survival were high but varied by sex and age: some females died from predation during incubation, but overall survival rates were very high during the breeding season with most mortality occurring during migration or during the winter (Table 2). We discuss the results in terms of (1) low observed dispersal rate even for chicks, (2) relatively high survival rates overall for a small passerine migrant (3) higher survival rates for males versus females and (4) lower survival rates for first-year birds. These four results are then discussed in terms of informing our understanding of the population dynamics of Afro–Palearctic migrants in general.
Dispersal rates
Observed dispersal rates in our study were relatively low compared to other studies of migrant passerines, but depended on age and sex with females and yearlings dispersing more than males (Table 3). This is consistent with other studies; for example, natal dispersal in the Collared Flycatcher Ficedula albicollis involved short distances with most young returning to breed within one kilometre of their birth site, and females dispersed further than males (Part 1990). Kern et al. (2014), showed that in Pied Flycatchers Ficedula hypoleuca, the overall return rate for males was 53 %, for females 42 % and 3 % for nestlings, thus suggesting that juveniles settled significantly farther away from their natal sites than adults, and females further away than males. The evolution of the Cyprus wheatear as an island endemic will have inevitably been associated with selection against long dispersal distance. Other island endemics also have relatively small dispersal distances (Bertrand et al. 2014; Wheelwright and Mauck 1998).
First-year Cyprus wheatears commonly dispersed from their natal site (90 % moved) at the scale of a few 100 m (most commonly, 3 territories away), whereas adults, if they moved at all (only 13 % moved), most commonly moved just 1 territory (Fig. 3). First-year birds typically disperse from their natal territory (Morton 1992) and this is primarily thought to be to avoid inbreeding (Doligez and Part 2008). For example, first-year Northern wheatears have been shown to disperse further than adults (Fulton 2010; Seward et al. 2013). Female chicks are generally found to be less philopatric and to disperse further than males (Greenwood and Harvey 1982). We found no evidence for this with Cyprus wheatear chicks: although our sample size was not large (N = 24 chicks), there was no indication of females moving a greater distance than males, and the trend was for females to move slightly less (half a territory). There was also no difference in dispersal rate observed for adult females compared to adult males (Fig. 2). This seems unlikely in terms of results from other studies; for example, Northern wheatears females disperse a greater distance than males (Arlt and Part 2008), and detecting female (or indeed any) dispersal depends crucially on the scale of a study (Doligez and Part 2008).
We noted a lower apparent overwinter survival rate for females (Table 4): this could have arisen because females had a bimodal distribution of dispersal distance. Some females dispersed close to their last breeding territory in a similar way to all males (accounting for our lack of a difference), whereas other females moved a larger distance away (and were not recorded because this distance exceeded the limits of our study area). Three pieces of evidence suggest that this may be the case. First, we know of one case where a female moved 1.3 km within a season to breed again, after initial failure, outside the study area. Second, considering all territories monitored in all 3 years with both male and female of a pair colour-ringed, there were 33 territories where both birds did not return, 10 where both birds returned, 13 where only the male returned and 6 where the only female returned. If female survival and dispersal is the same as a male's then we would expect equal numbers of territories in each class. We do observe similar numbers for territories with both and for just males returning, but much lower numbers for females. More data are needed in future years to test this statistically, but this strongly suggests that females are more likely to move territories if they come back and find their mate from last year has not returned, whereas males stay put. That males can sing and attract a mate, whereas females probably have to search for a mate, may then account for the sex-specific difference in staying or going if only one of a pair returns to a territory. This effect may be enhanced by the fact that females of many migrants arrive back on the breeding grounds later than males (Dierschke et al. 2005), so that a female’s opportunity of occupying the territory they occupied last year, if their partner from the previous year has not returned, may be constrained by its current occupation by an unsuitable male (Arlt and Part 2008). Third, the sex ratio of returned chicks was male rather than female biased (71 % male) when mortality (see below) and other studies suggest the reverse should be true (Donald 2007) if unconfounded by sex-biased dispersal.
Overall survival rates
The results indicate a very high survival rate for a small passerine migrant, with a minimum of 77 % of males surviving in some years (Table 4; Fig. 4). This is one of the highest survival rates recorded for any passerine migrant (e.g. Cuadrado et al. 1995; Grüebler et al. 2014). Overwinter survival rates measured at Troodos are likely to reflect those over the whole of Cyprus because the scale of migratory connectivity established for similar passerines means that Troodos breeding birds are likely to occupy the full wintering and migratory range of the species (Cresswell 2014) and Cyprus wheatears arrive well before and leave well after the finish of breeding throughout Cyprus (Flint and Stewart 1992 and pers. obs.), suggesting similar migration phenology. Similar high overwinter survival has been reported for both American Redstarts Setophaga ruticilla and Black-throated Blue Warblers Dendroica caerulescens with rates of up to 80 and 66 %, respectively (Holmes et al. 1989)—but this is only within the winter period and not including migration and breeding as in our estimates. Therefore, if migration costs are minimised, then survival of a passerine migrant wintering in the tropics, as is the case with Cyprus wheatears, can be high. Cyprus wheatears are relatively short distance, trans-Saharan migrants with a flight distance of about 2500 km to southern Sudan or Ethiopia (although exact wintering grounds information are limited: six birds from the study site recovered with geolocators in 2015 wintered in these areas, unpublished data). Although survival rate information for most Palearctic migrants is limited, populations of long-distance migrants in Europe are declining the most, suggesting migration distance influences survival rate (Sanderson et al. 2006; Jones and Cresswell 2010).
Another reason for such high survival rates may be because our study can measure true survival rather than apparent survival because of our low dispersal rates, particularly for males. Dispersal in many other populations of migrants is likely to be much higher and, indeed, in some populations, dispersal may lead to very low apparent survival rates (e.g. Shitikov et al. 2013). Even so, studies of passerine migrant survival that have considered dispersal have resulted in much lower survival rates than we report here (e.g. Cuadrado et al. 1995; Grüebler et al. 2014).
A final possible reason for high survival, at least during the period of time that Cyprus wheatears are on Cyprus, is the scarcity of predators that might prey on juvenile or adult birds at our study site on Troodos. In 4 years of intense observations (>500 h at the study site by WC who has studied sparrowhawks and the alarm calls they elicit elsewhere in Europe), no sparrowhawks Accipiter nisus or other likely woodland avian predator were observed on the study site, and most convincingly, no alarm calls associated with the presence of avian predators were heard from any of the species on the study area that commonly give alarm calls to sparrowhawks elsewhere in Europe such as Coal Tits Periparus ater, Chaffinches Fringilla coelebs, Blackbirds Turdus merula and Jay Garrulus glandarius (pers. obs.). We made only two observations in 4 years of Goshawks Accipiter nisus flying high overhead, indicating their rarity and, so, the low risk they must present. Other avian predators seen included regular Eleonora’s Falcons Falco eleonorae, but these were never seen below the canopy of the forest and only to hunt large aerial insects at Troodos. There were high densities of Scops Owls Otus scops, which, again, elicited no behavioural anti-predation response from Cyprus wheatears or other bird species whatsoever and, so, they were very unlikely to have represented a risk.
Sex-dependent survival rates
We found that female Cyprus wheatears had a higher mortality rate during the breeding season, with several females being killed during incubation in the nest. Because females are the sole incubators in Cyprus wheatears (Randler et al. 2010) and nested in cavities among rocks on the ground, they exposed themselves to ground predators such as snakes, agamid lizards, foxes, dogs and possibly even rats that were present on the study area; the remains of five of the seven females that disappeared during the breeding season were found on or very close to their nests, which also had been depredated or destroyed. Nest predation is the primary source of nestling mortality and can potentially shape avian breeding habitat preferences and life history strategies that reduce predator impacts on nestling survival (Martin 1995).
We also observed differences in apparent survival rates between adult male and female Cyprus wheatears, with females consistently showing a lower return rate than males. Some of this may be due to differences in dispersal, as discussed above, where underestimates of survival rate arise for females, because of a female bias in between-year breeding site fidelity (Clarke et al. 1997), especially among females with lower reproductive success (Hoover 2003). Nevertheless, lower annual survival rates for females can arise through carry-over effects because females may invest more in breeding attempts and care of young, as demonstrated by the Northern wheatear (Low et al. 2010), or because females can be disproportionately relegated to suboptimal habitats in winter by behavioural dominance of male conspecifics, as shown in American Redstarts (Marra and Holmes 2001). It should also be noted that although male Cyprus wheatears are apparently more vulnerable to predation risk because of their plumage and use of prominent song perches, making them more conspicuous, experimental studies on similar species, for example, Pied Flycatchers F. hypoleuca (Götmark 1995; Post and Gotmark 2006), have shown that the reverse is true. This may be because females cannot afford to allocate as much time to anti-predation behaviour in the limited time they have to forage when not nest-building or incubating. The lack of Accipiter hawks at the study site, however, potentially makes this issue less applicable.
Age-dependent survival rates
After accounting for dispersal, chicks had a lower overwinter survival than adults (chicks 13–16 % less than adult males). Note that we estimate survival for chicks after the first month after fledging when chick mortality is much higher in many passerine species (Anders et al. 1997), and, so, any differences between adults and first years are associated with their first migration to and from Africa and their first wintering period in Africa. Birds in their first year have been found, generally, to have lower survival than older birds (e.g. Saether 1989; Saether and Bakke 2000; Donovan et al. 1995).
Survival and the population dynamics of Afro–Palearctic migrants
Although the survival rates we recorded were relatively high, they were probably sufficiently annually variable to profoundly affect the annual population dynamics of Cyprus wheatears (see Baillie and Peach 1992; Newton 1998). In the case of the Cyprus wheatear at Troodos, survival rates are unarguably very high and must mean that this population has very few conservation issues both in terms of migration and wintering ground. Nevertheless, survival in Cyprus wheatears could vary by c. 30 % between years. Assuming an average 57 % survival for adults (mean of all yearly minimum true survival values for males and females) and typical productivity of 4 young per season (Xenophontos and Cresswell 2016) alive to migrate with a 49 % survival rate (mean of all yearly minimum true values for first years), this means that the population will have an average annual growth rate of 155 %, with a minimum growth rate (−15 % survival rate from average rates) of 110 % and a maximum growth rate (+15 % survival rate from average rates) of 200 %. The rate of population growth, therefore, varies by 1.8 dependent on survival rate. If survival rates were lower, this would make the difference between a declining or increasing population. Reducing average survival rates in both age classes by 4 % brings the poorest years to an approximate breakeven point, and reducing survival rate by 18 % in all cases brings the population to average stability.
Although survival rate for a Cyprus wheatear is high, it is also annually variable and probably affected by environmental factors mainly along the migration route and/or at non-breeding ground, with consequent strong annual effects on population dynamics. Future research for Cyprus wheatears should be focused on identifying sources of non-breeding mortality (extreme weather events during migration and wintering stage, predation and habitat destruction) which might strongly impact the annual survival of the species.
References
Anders AD, Dearborn DC, Faaborg J, Thompson FR (1997) Juvenile survival in a population of neotropical migrant birds. Conserv Biol 11:698–707
Arlt D, Part T (2008) Post-breeding information gathering and breeding territory shifts in northern wheatears. J Anim Ecol 77:211–219
Arlt D, Forslund P, Jeppsson T, Part T (2008) Habitat-specific population growth of a farmland bird. PLoS One. doi:10.1371/journal.pone.0003006
Baillie SR, Peach WJ (1992) Population limitation in Palearctic-African passerine migrants. Ibis 134(suppl. 1):120–132
Bertrand JAM, Bourgeois YXC, Delahaie B, Duval T, Garcia-Jimenez R, Cornuault J, Heeb P, Mila B, Pujol B, Thebaud C (2014) Extremely reduced dispersal and gene flow in an island bird. Heredity 112:190–196
Both C, te Marvelde L (2007) Climate change and timing of avian breeding and migration throughout Europe. Clim Res 35:93–105
Brasher MG, Arnold TW, Devries JH, Kaminski RM (2006) Breeding-season survival of male and female mallards in Canada’s prairie-parklands. J Wildl Manag 70:805–811
Clarke AL, Saether BE, Roskaft E (1997) Sex biases in avian dispersal: a reappraisal. Oikos 79:429–438
Clobert J, Perrins CM, McCleery RH, Gosler AG (1988) Survival rate in the great tit Parus-major in relation to sex, age, and immigration status. J Anim Ecol 57:287–306
Cresswell W (1994) Age-dependent choice of redshank (Tringa totanus) feeding location: Profitability or risk? J Anim Ecol 63:589–600
Cresswell W (2014) Migratory connectivity of Palaearctic-African migratory birds and their responses to environmental change: the serial residency hypothesis. Ibis 156:493–510
Cuadrado M, Senar JC, Copete JL (1995) Do all blackcaps Sylvia atricapilla show winter site fidelity? Ibis 137:70–75
Desrochers A (1992) Age and foraging success in European blackbirds: variation between and within individuals. Anim Behav 43:885–894
Dierschke V, Mendel B, Schmaljohann H (2005) Differential timing of spring migration in northern wheatears Oenanthe oenanthe: Hurried males or weak females? Behav Ecol Sociobiol 57:470–480
Doligez B, Part T (2008) Estimating fitness consequences of dispersal: A road to ‘know-where’? Non-random dispersal and the underestimation of dispersers’ fitness. J Anim Ecol 77:1199–1211
Donald PF (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Donovan TM, Thompson FR III, Faaborg J, Probst JR (1995) Reproductive success of migratory birds in habitat sources and sinks. Conserv Biol 9:1380–1395
Dukas R, Kamil AC (2000) The cost of limited attention in blue jays. Behav Ecol 11:502–506
Flint PR, Stewart PF (1992) The birds of Cyprus. British Ornithologists Union, Tring
Fulton D (2010) The breeding population of northern wheatears at Clee Hill, Shropshire, 1998–2009. Br Birds 103:223–228
Gardali T, Barton DC, White JD, Geupel GR (2003) Juvenile and adult survival of Swainson’s Thrush (Catharus ustulatus) in coastal California: annual estimates using capture-recapture analyses. Auk 120:1188–1194
Götmark F (1995) Black-and-white plumage in male pied flycatchers (Ficedula hypoleuca) reduces the risk of predation from sparrowhawks (Accipiter nisus) during the breeding season. Behav Ecol 6:22–26
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Ann Rev Ecol Syst 13:1–21
Grubler MU, Schuler H, Muller M, Spaar R, Horch P, Naef-Daenzer B (2008) Female biased mortality caused by anthropogenic nest loss contributes to population decline and adult sex ratio of a meadow bird. Biol Conserv 141:3040–3049
Grüebler MU, Korner-Nievergelt F, Naef-Daenzer B (2014) Equal nonbreeding period survival in adults and juveniles of a long-distant migrant bird. Ecol Evol 4:756–765
Holmes RT, Sherry TW, Reitsma L (1989) Population structure, territoriality and overwinter survival of 2 migrant warbler species in Jamaica. Condor 91:545–561
Hoover JP (2003) Decision rules for site fidelity in a migratory bird, the prothonotary warbler. Ecology 84:416–430
Jakobsson S, Brick O, Kullberg C (1995) Escalated fighting behaviour incurs increased predation risk. Anim Behav 49:235–239
Jones T, Cresswell W (2010) The phenology mismatch hypothesis: Are declines of migrant birds linked to uneven global climate change? J Anim Ecol 79:98–108
Kanyamibwa S, Bairlein F, Schierer A (1993) Comparison of survival rates between populations of the white stork Ciconia-ciconia in central-Europe. Ornis Scand 24:297–302
Kern M, Slater F, Cowie R (2014) Return rates and dispersal distances of Welsh Ped Flycatchers Ficedula hypoleuca and factors that influence them. Ring Migr 29:1–9
Krams I, Vrublevska J, Koosa K, Krama T, Mierauskas P, Rantala MJ, Tilgar V (2014) Hissing calls improve survival in incubating female great tits (Parus major). Acta Ethol 17:83–88
Low M, Arlt D, Eggers S, Part T (2010) Habitat-specific differences in adult survival rates and its links to parental workload and on-nest predation. J Anim Ecol 79:214–224
Marra PP, Holmes RT (2001) Consequence of dominance-mediated habitat segregation in American redstarts during the non-breeding season. Auk 118:92–104
Martin TE (1995) Avian life history evolution in relation to nest sites, nest predation and food. Ecol Monogr 65:101–127
McCleery RH, Clobert J, Julliard R, Perrins CM (1996) Nest predation and delayed cost of reproduction in the great tit. J Anim Ecol 65:96–104
Morton ML (1992) Effects of sex and birth date on premigration biology, migration schedules, return rates and natal dispersal in the mountain white-crowned sparrow. Condor 94:117–133
Newton I (1998) Population limitation in birds. Academic Press, London
Newton I (2004) Population limitation in migrants. Ibis 146:197–226
Newton I (2008) The migration ecology of birds. Academic Press, Oxford
Ockendon N, Hewson CM, Johnston A, Atkinson PW (2012) Declines in British-breeding populations of Afro-Palaearctic migrant birds are linked to bioclimatic wintering zone in Africa, possibly via constraints on arrival time advancement. Bird Study 59:111–125
Part T (1990) Natal dispersal in the collared flycatcher—possible causes and reproductive consequences. Ornis Scand 21:83–88
Peach WJ, Siriwardena GM, Gregory RD (1999) Long-term changes in over-winter survival rates explain the decline of reed buntings Emberiza schoeniclus in Britain. J Appl Ecol 36:798–811
Perlut NG, Strong AM, Donovan TM, Buckley NJ (2008) Regional population viability of grassland songbirds: effects of agricultural management. Biol Conserv 141:3139–3151
Post P, Gotmark F (2006) Predation by sparrowhawks Accipiter nisus on male and female pied flycatchers Ficedula hypoleuca in relation to their breeding behaviour and foraging. J Av Biol 37:158–168
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Randler C, Teichmann C, Pentzold S (2010) Breeding habitat preference and foraging of the Cyprus wheatear Oenanthe cypriaca and niche partitioning in comparison with migrant Oenanthe species on Cyprus. J Ornith 151:113–121
Randler C, Foerschler MI, Gonzalez J, Aliabadian M, Bairlein F, Wink M (2012) Phylogeography, pre-zygotic isolation and taxonomic status in the endemic Cyprus wheatear Oenanthe cypriaca. J Ornith 153:303–312
Robinson RA, Baillie SR, Crick HQP (2007) Weather-dependent survival: implications of climate change for passerine population processes. Ibis 149:357–364
Saether BE (1989) Survival rates in relation to body-weight in European birds. Ornis Scand 20:13–21
Saether BE, Bakke O (2000) Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81:642–653
Saether BE, Engen S (2002) Pattern of variation in avian population growth rates. PhilTransRSocLondSerB 357:1185–1195
Saino N, Szep T, Romano M, Rubolini D, Spina F, Moller AP (2004) Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol Lett 7:21–25
Sanderson FJ, Donald PF, Pain DJ, Burfield IJ, van Bommel FPJ (2006) Long-term population declines in Afro-Palearctic migrant birds. Biol Conserv 131:93–105
Schaub M, Kania W, Koppen U (2005) Variation of primary production during winter induces synchrony in survival rates in migratory white storks Ciconia ciconia. J Anim Ecol 74:656–666
Schubert KA, Mennill DJ, Ramsay SM, Otter KA, Ratcliffe LM, Kraus C (2008) Between-year survival and rank transitions in male Black-capped Chickadees (Poecile atricapillus): a multistate modeling approach. Auk 125:629–636
Seward AM, Beale CM, Gilbert L, Jones TH, Thomas RJ (2013) The impact of increased food availability on survival of a long-distance migratory bird. Ecology 94:221–230
Shitikov DA, Dubkova EV, Makarova TV (2013) The demography of Yellow Wagtails Motacilla flava on abandoned fields in northern European Russia. Bird Study 60:518–526
Sillett TS, Holmes RT (2002) Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol 71:296–308
Strandberg R, Klaassen RHG, Hake M, Alerstam T (2010) How hazardous is the Sahara Desert crossing for migratory birds? Indications from satellite tracking of raptors. Biol Lett UK 6:297–300
Thaxter CB, Joys AC, Gregory RD, Baillie SR, Noble DG (2010) Hypotheses to explain patterns of population change among breeding bird species in England. Biol Conserv 143:2006–2019
Wheelwright NT, Mauck RA (1998) Philopatry, natal dispersal, and inbreeding avoidance in an island population of Savannah Sparrows. Ecology 79:755–767
Witter MS, Cuthill IC, Bonser RHC (1994) Experimental investigations of mass-dependent predation risk in the European Starling, Sturnus vulgaris. Anim Behav 48:201–222
Xenophontos M, Cresswell W (2016) Reproductive success and productivity of the Cyprus Wheatear Oenanthe cypriaca, a migratory, island endemic. J Ornithol. doi:10.1007/s10336-015-1322-2
Zuk M, Kolluru GR (1998) Exploitation of sexual signals by predators and parasitoids. Q Rev Biol 73:415–438
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xenophontos, M., Cresswell, W. Survival and dispersal of the Cyprus wheatear Oenanthe cypriaca, an endemic migrant. J Ornithol 157, 707–719 (2016). https://doi.org/10.1007/s10336-015-1315-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1315-1