Abstract
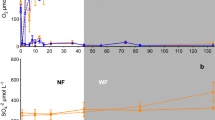
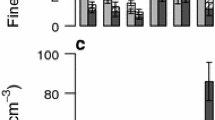
Iron (Fe) reduction and oxidation are important biogeochemical processes coupled to decomposition, nutrient cycling, and mineral weathering, but factors controlling their rates and spatial distribution with depth are poorly understood in terrestrial soils. In aquatic ecosystems, Fe reduction often occurs below a zone of oxic sediments. We tested an alternative conceptual model for Fe redox cycling in terrestrial soils using a deep humid tropical forest soil profile. We hypothesized that Fe reduction in anaerobic microsites scales with depth variation in labile C and Fe availability, as opposed to bulk oxygen (O2). We measured bulk O2 at multiple depths from 0.1 to 5 m quasi-continuously over 18 months and sampled soils from surface to bedrock (~7 m). Median O2 mixing ratios declined from 19.8 ± 1.2 % at 0.25 m to 16.1 ± 1.0 % at 1 m, but did not consistently decrease below 1 m, challenging a recent model of regolith development. Reduced Fe (Fe(II)) extractable in 0.5 M hydrochloric acid was greatest in 0–0.1 m soil and declined precipitously with depth, and did not correspond with visible gleying in B horizons. We observed similar depth trends in potential Fe reduction under anaerobic conditions. Depth trends in Fe(II) also closely mirrored short-term soil respiration and bulk soil C. Labile C stimulated Fe reduction at 0–0.1 m depth, whereas addition of short-range-ordered Fe oxides had no effect. Cultivable Fe-reducing bacterial abundance was four orders of magnitude greater in surface soil (0–0.1 m) than below 1 m. Although cultivable Fe oxidizing bacteria were typically also more abundant in surface soil, addition of labile C and nitrate stimulated Fe oxidizers in deep soil by two orders of magnitude under anaerobic conditions. This implies that infiltration of nitrate (and possibly C) from shallow soil water could potentially promote biotic Fe oxidation, a critical step in bedrock weathering, 7 m below. Together, these data suggest that C, Fe, and nutrient availability increase microbial Fe reduction and oxidation in surface (vs deeper) soil microsites despite high bulk O2, in contrast to the depth segregation of electron accepting processes often observed in aquatic ecosystems. Furthermore, the greatest capacity for Fe redox cycling can occur in A horizons that do not display gleying or mottling.





Similar content being viewed by others
References
Bazilevskaya E, Lebedeva M, Pavich M et al (2013) Where fast weathering creates thin regolith and slow weathering creates thick regolith. Earth Surf Process Landf 38:847–858. doi:10.1002/esp.3369
Behrens R, Bouchez J, Schuessler JA et al (2015) Mineralogical transformations set slow weathering rates in low-porosity metamorphic bedrock on mountain slopes in a tropical climate. Chem Geol 411:283–298. doi:10.1016/j.chemgeo.2015.07.008
Böhlke JK, Wanty R, Tuttle M et al (2002) Denitrification in the recharge area and discharge area of a transient agricultural nitrate plume in a glacial outwash sand aquifer, Minnesota. Water Resour Res 38:10–11. doi:10.1029/2001WR000663
Bonneville S, Behrends T, Van Cappellen P (2009) Solubility and dissimilatory reduction kinetics of iron(III) oxyhydroxides: a linear free energy relationship. Geochim Cosmochim Acta 73:5273–5282. doi:10.1016/j.gca.2009.06.006
Bowling DR, Egan JE, Hall SJ, Risk DA (2015) Environmental forcing does not induce diel or synoptic variation in the carbon isotope content of forest soil respiration. Biogeosciences 12:5143–5160. doi:10.5194/bg-12-5143-2015
Brantley SL, White AF (2009) Approaches to modeling weathered regolith. Rev Miner Geochem 70:435–484. doi:10.2138/rmg.2009.70.10
Buettner SW, Kramer MG, Chadwick OA, Thompson A (2014) Mobilization of colloidal carbon during iron reduction in basaltic soils. Geoderma 221–222:139–145. doi:10.1016/j.geoderma.2014.01.012
Buss HL, Bruns MA, Schultz MJ et al (2005) The coupling of biological iron cycling and mineral weathering during saprolite formation, Luquillo Mountains, Puerto Rico. Geobiology 3:247–260. doi:10.1111/j.1472-4669.2006.00058.x
Buss HL, Mathur R, White AF, Brantley SL (2010) Phosphorus and iron cycling in deep saprolite, Luquillo Mountains, Puerto Rico. Chem Geol 269:52–61. doi:10.1016/j.chemgeo.2009.08.001
Cerling TE (1991) Carbon dioxide in the atmosphere; evidence from cenozoic and mesozoic paleosols. Am J Sci 291:377–400. doi:10.2475/ajs.291.4.377
Chacon N, Silver WL, Dubinsky EA, Cusack DF (2006) Iron reduction and soil phosphorus solubilization in humid tropical forest soils: the roles of labile carbon pools and an electron shuttle compound. Biogeochemistry 78:67–84. doi:10.1007/s10533-005-2343-3
Chapelle FH, McMahon PB, Dubrovsky NM et al (1995) Deducing the distribution of terminal electron-accepting processes in hydrologically diverse groundwater systems. Water Resour Res 31:359–371
Cheng L, Zhu J, Chen G et al (2010) Atmospheric CO2 enrichment facilitates cation release from soil. Ecol Lett 13:284–291. doi:10.1111/j.1461-0248.2009.01421.x
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci 103:10316–10321. doi:10.1073/pnas.0600989103
Cusack DF, Silver WL, Torn MS, McDowell WH (2011) Effects of nitrogen additions on above- and belowground carbon dynamics in two tropical forests. Biogeochemistry 104:203–225. doi:10.1007/s10533-010-9496-4
DeAngelis KM, Silver WL, Thompson AW, Firestone MK (2010) Microbial communities acclimate to recurring changes in soil redox potential status. Environ Microbiol 12:3137–3149. doi:10.1111/j.1462-2920.2010.02286.x
Dubinsky EA, Silver WL, Firestone MK (2010) Tropical forest soil microbial communities couple iron and carbon biogeochemistry. Ecology 91:2604–2612. doi:10.1890/09-1365.1
Emerson S, Hedges J (2003) Sediment diagenesis and benthic flux. Treatise Geochem 6:293–319. doi:10.1016/B0-08-043751-6/06112-0
Fimmen RL, deB Richter D, Vasudevan D et al (2008) Rhizogenic Fe–C redox cycling: a hypothetical biogeochemical mechanism that drives crustal weathering in upland soils. Biogeochemistry 87:127–141
Fletcher RC, Buss HL, Brantley SL (2006) A spheroidal weathering model coupling porewater chemistry to soil thicknesses during steady-state denudation. Earth Planet Sci Lett 244:444–457. doi:10.1016/j.epsl.2006.01.055
Froelich PN, Klinkhammer GP, Bender ML et al (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim Cosmochim Acta 43:1075–1090. doi:10.1016/0016-7037(79)90095-4
Fuss CB, Driscoll CT, Johnson CE et al (2010) Dynamics of oxidized and reduced iron in a northern hardwood forest. Biogeochemistry 104:103–119. doi:10.1007/s10533-010-9490-x
Hall SJ, Silver WL (2013) Iron oxidation stimulates organic matter decomposition in humid tropical forest soils. Glob Change Biol 19:2804–2813. doi:10.1111/gcb.12229
Hall SJ, Silver WL (2015) Reducing conditions, reactive metals, and their interactions can explain spatial patterns of surface soil carbon in a humid tropical forest. Biogeochemistry 125:149–165. doi:10.1007/s10533-015-0120-5
Hall SJ, McDowell WH, Silver WL (2013) When wet gets wetter: decoupling of moisture, redox biogeochemistry, and greenhouse gas fluxes in a humid tropical forest soil. Ecosystems 16:576–589. doi:10.1007/s10021-012-9631-2
Hall SJ, Silver WL, Timokhin VI, Hammel KE (2016) Iron addition to soil specifically stabilized lignin. Soil Biol Biochem 98:95–98. doi:10.1016/j.soilbio.2016.04.010
Hyacinthe C, Bonneville S, Van Cappellen P (2006) Reactive iron(III) in sediments: chemical versus microbial extractions. Geochim Cosmochim Acta 70:4166–4180. doi:10.1016/j.gca.2006.05.018
Jeon B-H, Dempsey BA, Burgos WD (2003) Kinetics and mechanisms for reactions of Fe(II) with iron(III) oxides. Environ Sci Technol 37:3309–3315. doi:10.1021/es025900p
Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436. doi:10.1890/1051-0761(2000)010
Johnson AH, Xing HX, Scatena FN (2015) Controls on soil carbon stocks in El Yunque National Forest, Puerto Rico. Soil Sci Soc Am J 79:294. doi:10.2136/sssaj2014.05.0199
Kappler A, Schink B, Newman DK (2005) Fe(III) mineral formation and cell encrustation by the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology 3:235–245. doi:10.1111/j.1472-4669.2006.00056.x
Keiluweit M, Nico PS, Kleber M, Fendorf S (2016) Are oxygen limitations under recognized regulators of organic carbon turnover in upland soils? Biogeochemistry 127(2–3):157–171. doi:10.1007/s10533-015-0180-6
Klee AJ (1996) Most probable number calculator. US environmental protection agency, risk reduction engineering laboratory, Cincinnati
Liermann LJ, Albert I, Buss HL et al (2015) Relating microbial community structure and geochemistry in deep regolith developed on volcaniclastic rock in the Luquillo Mountains, Puerto Rico. Geomicrobiol J 32:494–510. doi:10.1080/01490451.2014.964885
Liptzin D, Silver WL (2015) Spatial patterns in oxygen and redox sensitive biogeochemistry in tropical forest soils. Ecosphere 6:1–14. doi:10.1890/ES14-00309.1
Liptzin D, Silver WL, Detto M (2011) Temporal dynamics in soil oxygen and greenhouse gases in two humid tropical forests. Ecosystems 14:171–182. doi:10.1007/s10021-010-9402-x
Lovley DR (1995) Microbial reduction of iron, manganese, and other metals. Adv Agron 54:175–231
Melton ED, Swanner ED, Behrens S et al (2014) The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12:797–808. doi:10.1038/nrmicro3347
Nepstad DC, de Carvalho CR, Davidson EA et al (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:666–669. doi:10.1038/372666a0
Norkko J, Reed DC, Timmermann K et al (2011) A welcome can of worms? Hypoxia mitigation by an invasive species. Glob Change Biol 18:422–434. doi:10.1111/j.1365-2486.2011.02513.x
Peretyazhko T, Sposito G (2005) Iron(III) reduction and phosphorous solubilization in humid tropical forest soils. Geochim Cosmochim Acta 69:3643–3652. doi:10.1016/j.gca.2005.03.045
Pfennig N, Trüper HG (1992) The family Chromatiaceae. In: Balows A, Trüper HG, Dworkin M et al (eds) The prokaryotes. Springer, New York, pp 3200–3221
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44:81–99. doi:10.1034/j.1600-0889.1992.t01-1-00001.x
Roden E, Wetzel R (1996) Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr 41:1733–1748
Roden EE, Wetzel RG (2002) Kinetics of microbial Fe(III) oxide reduction in freshwater wetland sediments. Limnol Oceanogr 47:198–211
Roden EE, Zachara JM (1996) Microbial reduction of crystalline Iron(III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol 30:1618–1628. doi:10.1021/es9506216
Sanchez PA (1976) Properties and management of soils in the tropics. Wiley, New York
Schulz MS, White AF (1999) Chemical weathering in a tropical watershed, Luquillo Mountains, Puerto Rico III: quartz dissolution rates. Geochim Cosmochim Acta 63:337–350. doi:10.1016/S0016-7037(99)00056-3
Schulz M, Stonestrom D, Lawrence C, et al (2016) Structured heterogeneity in a marine terrace chronosequence: upland mottling. Vadose Zone J 15:0. doi: 10.2136/vzj2015.07.0102
Schwertmann U, Murad E (1988) The nature of an iron oxide-organic iron association in a peaty environment. Clay Miner 23:291–299
Seitzinger S, Harrison JA, Böhlke JK et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090. doi:10.1890/1051-0761(2006)016
Sexstone A, Revsbech N, Parkin T, Tiedje J (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J 49:645–651
Silver WL, Lugo AE, Keller M (1999) Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 44:301–328. doi:10.1023/A:1006034126698
Silver WL, Liptzin D, Almaraz M (2013) Soil redox dynamics and biogeochemistry along a tropical elevation gradient. In: Gonzalez G, Willig MR, Waide RB (eds) Ecological gradient analyses in a tropical landscape. Wiley, New Jersey
Soil Survey Staff (2002) Soil survey of caribbean national forest and Luquillo experimental forest, commonwealth of Puerto Rico. United States department of agriculture, natural resources conservation service, Washingtion, D. C
Solomon DK, Cerling TE (1987) The annual carbon dioxide cycle in a montane soil: observations, modeling, and implications for weathering. Water Resour Res 23:2257–2265. doi:10.1029/WR023i012p02257
Stone MM, DeForest JL, Plante AF (2014) Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo critical zone observatory. Soil Biol Biochem 75:237–247. doi:10.1016/j.soilbio.2014.04.017
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62:1458–1460
Thamdrup B (2000) Bacterial manganese and iron reduction in aquatic sediments. In: Schink B (ed) Advances in Microbial Ecology. Springer, Boston, pp 41–84
Thompson A, Chadwick OA, Boman S, Chorover J (2006) Colloid mobilization during soil iron redox oscillations. Environ Sci Technol 40:5743–5749. doi:10.1021/es061203b
Thompson A, Rancourt D, Chadwick O, Chorover J (2011) Iron solid-phase differentiation along a redox gradient in basaltic soils. Geochim Cosmochim Acta 75:119–133. doi:10.1016/j.gca.2010.10.005
Veneman PLM, Vepraskas MJ, Bouma J (1976) The physical significance of soil mottling in a Wisconsin toposequence. Geoderma 15:103–118. doi:10.1016/0016-7061(76)90081-1
Viollier E, Inglett P, Hunter K et al (2000) The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem 15:785–790. doi:10.1016/S0883-2927(99)00097-9
von Fischer JC, Hedin LO (2007) Controls on soil methane fluxes: tests of biophysical mechanisms using stable isotope tracers. Glob Biogeochem Cycle 21:9. doi:10.1029/2006gb002687
Weaver PL, Murphy PG (1990) Forest structure and productivity in Puerto Rico’s Luquillo Mountains. Biotropica 22:69–82. doi:10.2307/2388721
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764. doi:10.1038/nrmicro1490
White AF, Blum AE, Schulz MS et al (1998) Chemical weathering in a tropical watershed, Luquillo Mountains, Puerto Rico: I. Long-term versus short-term weathering fluxes. Geochim Cosmochim Acta 62:209–226. doi:10.1016/S0016-7037(97)00335-9
Widdel F, Bak F (1992) Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper HG, Dworkin M et al (eds) The prokaryotes. Springer, New York, pp 3352–3378
Yang WH, Liptzin D (2015) High potential for iron reduction in upland soils. Ecology 96:2015–2020. doi:10.1890/14-2097.1
Yang WH, Weber KA, Silver WL (2012) Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction. Nat Geosci 5:538–541. doi:10.1038/ngeo1530
Yi-Balan SA, Amundson R, Buss HL (2014) Decoupling of sulfur and nitrogen cycling due to biotic processes in a tropical rainforest. Geochim Cosmochim Acta 142:411–428. doi:10.1016/j.gca.2014.05.049
Acknowledgments
Data associated with this manuscript will be available on the Luquillo CZO data repository (http://criticalzone.org/luquillo/data/) after publication. We thank Heather Dang and Andrew McDowell for crucial help in the lab, and Manual Rosario for data collection. We thank Aaron Thompson for providing valuable insights on this work. This work was supported by NSF Grant DEB-1457805 to WLS and SJH, and the NSF Luquillo Critical Zone Observatory (EAR-0722476) and LTER (DEB-0620910).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Sasha C. Reed.
Rights and permissions
About this article
Cite this article
Hall, S.J., Liptzin, D., Buss, H.L. et al. Drivers and patterns of iron redox cycling from surface to bedrock in a deep tropical forest soil: a new conceptual model. Biogeochemistry 130, 177–190 (2016). https://doi.org/10.1007/s10533-016-0251-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-016-0251-3