Abstract
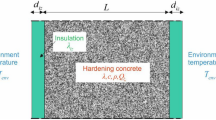
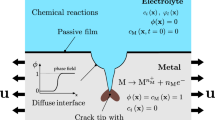
An alternative approach is developed to incorporate chloride binding/release processes during modeling of ingress of external chlorides in concrete through the use of thermodynamic modeling of chemical reactions. Transport of chloride in concrete is modeled through transient finite element analysis. At each time marching step, the chloride binding/release reactions are modeled using thermodynamic calculations. For this purpose, an open-source thermodynamic modeling software is used to model all possible reactions within the cementitious matrix including the reactions of chlorides with unhydrated and hydrated cementitious materials. The predictive ability of thermodynamic calculations is presented by comparing them with experimental data. The proposed and traditional modeling approaches for chloride transport with binding are compared. Finally, a parametric investigation is presented to demonstrate some of the strengths of the proposed approach using thermodynamic calculations over the traditional approach using binding isotherms to simulate chloride ingress in concrete.











Similar content being viewed by others
Notes
Cement notation is used. C3A: 3CaO·Al2O3
C4AF: 4CaO·Al2O3·Fe2O3
C–S–H: Stoichiometry varies; a typical composition is 0.8–1.5
CaO·SiO2·1.0–2.5 H2O.
References
Bertolini, L., Elsener, B., Pedeferri, P., Polder, R.: Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair. Wiley-VCH, Weinheim (2000)
Broomfield, J.P.: Corrosion of Steel in Concrete. Taylor & Francis, New York (2007)
Revie, R.W., Uhlig, H.H.: Corrosion and Corrosion Control, 4th edn. Wiley-Interscience, New York (2008)
Bohni, H.: Corrosion in Concrete Structures. CRC Press, New York (2005)
Kurtis, K.E., Mehta, P.K.: A critical review of deterioration of concrete due to corrosion of reinforcing steel. ACI Spec. Publ. 170, 535–554 (1997)
Mehta, P.K., Monteiro, P.J.M.: Concrete: Microstructure, Properties, and Materials. McGraw-Hill Professional, New York (2005)
Koch, G.H., Brongers, M., P.H., Thompson, N.G., Virmani, Y.P., Payer, J.H.: Corrosion cost and preventative strategies in the United States. In: NACE International (2003)
Samson, E., Marchand, J.: Modeling the transport of ions in unsaturated cement-based materials. Comput. Struct. 85(23–24), 1740–1756 (2007). doi:10.1016/j.compstruc.2007.04.008
Samson, E., Marchand, J.: Numerical solution of the extended Nernst–Planck model. J. Colloid Interface Sci. 215(1), 1–8 (1999). doi:10.1006/jcis.1999.6145
Azad, V.J., Li, C., Verba, C., Ideker, J.H., Isgor, O.B.: A COMSOL–GEMS interface for modeling coupled reactive-transport geochemical processes. Comput. Geosci. UK 92, 79–89 (2016)
Karadakis, K., Azad, V.J., Ghods, P., Isgor, O.B.: Numerical investigation of the role of mill scale crevices on the corrosion initiation of carbon steel reinforcement in concrete. J. Electrochem. Soc. 163(6), C306–C315 (2016)
Martin-Perez, B., Pantazopoulou, S.J., Thomas, M.D.A.: Numerical solution of mass transport equations in concrete structures. Comput. Struct. 79(13), 1251–1264 (2001). doi:10.1016/S0045-7949(01)00018-9
Isgor, O.B., Razaqpur, A.G.: Finite element modeling of coupled heat transfer, moisture transport and carbonation processes in concrete structures. Cem. Concr. Comp. 26(1), 57–73 (2004). doi:10.1016/S0958-9465(02)00125-7
van der Zanden, A.J.J., Taher, A., Arends, T.: Modelling of water and chloride transport in concrete during yearly wetting/drying cycles. Constr. Build. Mater. 81, 120–129 (2015)
Marchand, J., Samson, E.: Predicting the service-life of concrete structures—Limitations of simplified models. Cem. Concr. Comp. 31(8), 515–521 (2009). doi:10.1016/j.cemconcomp.2009.01.007
Florea, M.V.A., Brouwers, H.J.H.: Chloride binding related to hydration products Part I: ordinary portland cement. Cem. Concr. Res. 42(2), 282–290 (2012)
Yuan, Q., Shi, C.J., De Schutter, G., Audenaert, K., Deng, D.H.: Chloride binding of cement-based materials subjected to external chloride environment—a review. Constr. Build. Mater. 23(1), 1–13 (2009)
Birnin-Yauri, U.A., Glasser, F.P.: Friedel’s salt, Ca2Al(OH)(6)(Cl, OH)center dot 2H(2)O: its solid solutions and their role in chloride binding. Cem. Concr. Res. 28(12), 1713–1723 (1998)
Glasser, F.P., Kindness, A., Stronach, S.A.: Stability and solubility relationships in AFm phases—part 1. Chloride, sulfate and hydroxide. Cem. Concr. Res. 29(6), 861–866 (1999)
Suryavanshi, A.K., Scantlebury, J.D., Lyon, S.B.: Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 26(5), 717–727 (1996)
Plusquellec, G., Nonat, A.: Interactions between calcium silicate hydrate (C–S–H) and calcium chloride, bromide and nitrate. Cem. Concr. Res. 90, 89–96 (2016)
Martin-Perez, B., Zibara, H., Hooton, R.D., Thomas, M.D.A.: A study of the effect of chloride binding on service life predictions. Cem. Concr. Res. 30(8), 1215–1223 (2000)
Neville, A.M.: Properties of Concrete, 4th edn. Pearson Education Limited, Essex (1996)
Samson, E., Marchand, J., Robert, J.L., Bournzel, J.P.: Modelling ion diffusion mechanisms in porous media. Int. J. Numer. Meth. Eng. 46(12), 2043–2060 (1999)
Davies, C.: Electrochemistry, Newnes. In: London (1967)
Nilsson, L.O., Massat, M., Tang, L.: The effect of non-linear chloride binding on the prediction of chloride penetration into concrete structutres. In: Malhotra, V.M. (ed.) Durability of Concrete, pp. 469–486. American Concrete Institute (ACI), Detroit (1994)
Tang, L.P., Nilsson, L.O.: Chloride binding-capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 23(2), 247–253 (1993)
Kulik, D.A., Wagner, T., Dmytrieva, S.V., Kosakowski, G., Hingerl, F.F., Chudnenko, K.V., Berner, U.R.: GEM-selektor geochemical modeling package: revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 17(1), 1–24 (2013). doi:10.1007/s10596-012-9310-6
Wagner, T., Kulik, D.A., Hingerl, F.F., Dmytrieva, S.V.: Gem-selektor geochemical modeling package: TSolmod library and data interface for multicomponent phase models. Can. Miner. 50(5), 1173–1195 (2012). doi:10.3749/canmin.50.5.1173
Kulik, D., Berner, U., Curti, E.: Modelling chemical equilibrium partitioning with the GEMS-PSI code (2004)
Kulik, D.A.: Gibbs energy minimization approach to modeling sorption equilibria at the mineral-water interface: thermodynamic relations for multi-site-surface complexation. Am. J. Sci. 302(3), 227–279 (2002). doi:10.2475/ajs.302.3.227
Johnson, J.W., Oelkers, E.H., Helgeson, H.C.: SUPCRT92: a software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 C. Comput. Geosci. UK 18(7), 899–947 (1992)
Hummel, W., Berner, U., Curti, E., Pearson, F., Thoenen, T.: Nagra/PSI chemical thermodynamic data base. Radiochim. Acta 90(9–11), 805–813 (2002)
Lothenbach, B., Winnefeld, F.: Thermodynamic modelling of the hydration of portland cement. Cem. Concr. Res. 36(2), 209–226 (2006). doi:10.1016/j.cemconres.2005.03.001
Loser, R., Lothenbach, B., Leemann, A., Tuchschmid, M.: Chloride resistance of concrete and its binding capacity—comparison between experimental results and thermodynamic modeling. Cem. Concr. Comp. 32(1), 34–42 (2010)
Zibara, H.: Binding of External Chlorides by Cement Pastes. University of Toronto, Toronto (2001)
Powers, T.C., Brownyard, T.L.: Studies of the physical properties of hardened portland cement paste. J. Am. Concr. Inst. 18(3), 249–336 (1946)
Lumley, J.S., Gollop, R.S., Moir, G.K., Taylor, H.F.W.: Degrees of reaction of the slag ln some blends with portland cements. Cem. Concr. Res. 26(1), 139–151 (1996)
Zeng, Q., Li, K.F., Fen-chong, T., Dangla, P.: Determination of cement hydration and pozzolanic reaction extents for fly-ash cement pastes. Constr. Build. Mater. 27(1), 560–569 (2012)
Boddy, A., Bentz, E., Thomas, M.D.A., Hooton, R.D.: An overview and sensitivity study of a multimechanistic chloride transport model. Cem. Concr. Res. 29(6), 827–837 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azad, V.J., Isgor, O.B. Modeling chloride ingress in concrete with thermodynamically calculated chemical binding. Int J Adv Eng Sci Appl Math 9, 97–108 (2017). https://doi.org/10.1007/s12572-017-0189-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12572-017-0189-2