Summary
Although localized prostate cancer (PC) is a curable disease, a significant proportion of patients progress from advanced PC to metastatic castration-resistant prostate cancer (mCRPC). Several new treatments for mCRPC were approved including new hormonal therapies, chemotherapies, and radium-223. For patients with disease progression after the standard therapies, lutetium-prostate-specific membrane antigen (Lu-PSMA) therapy could be an option.
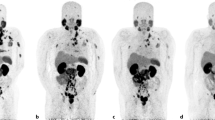
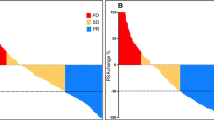
The aim of this short review is to give an overview of the available evidence of the efficacy and toxicity of Lu-PSMA therapy. To date, one prospective phase II trial and several small retrospective studies have been published including almost 400 patients. Primary endpoint of all available trials was a decline in PSA of >50%, which was achieved in 30–70% of men treated with Lu-PSMA therapy. A PSA decline was observed in 70–91% of cases. Overall survival was reported only in small retrospective subgroups, and no prospective data are available. Progressive disease was observed in 11–32% of patients after Lu-PSMA treatment.
Lu-PSMA treatment seems to be well tolerated and safe. One common treatment-related grade 1 adverse event is dry mouth in up to 87% of men after radioligand therapy (RLT). Therefore, in the vast majority no treatment is needed. Hematologic toxicity such as thrombocytopenia caused serious grade 3–4 adverse events in 27% of patients in the prospective trial. No immediate adverse events after radioligand infusion and no treatment-related deaths were reported.
According to preliminary data, Lu-PSMA therapy seems to be well tolerated and safe for progressive end-stage mCRPC patients. Further prospective trials are needed.
Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E86.
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12.
de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54.
de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23.
Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48.
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33.
Santoni M, Scarpelli M, Mazzucchelli R, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promises. J Biol Regul Homeost Agents. 2014;28(4):555–63.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81–5.
Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(12):2117–36.
Spatz S, Tolkach Y, Jung K, et al. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: implications for imaging studies and prognostic role. J Urol. 2018;199(2):370–7.
Kabasakal L, AbuQbeitah M, Aygun A, et al. Pre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(13):1976–83.
Wright GL Jr., Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1(1):18–28.
Hofman MS, Violet J, Hicks RJ, et al. (177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–33.
Kratochwil C, Giesel FL, Eder M, et al. (1)(7)(7)Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(6):987–8.
Ahmadzadehfar H, Eppard E, Kurpig S, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7(11):12477–88.
Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. Ejnmmi Res. 2015;5(1):114.
Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44(9):1448–54.
Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57(7):1006–13.
Brauer A, Grubert LS, Roll W, et al. (177)Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(10):1663–70.
Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57(8):1170–6.
Rahbar K, Bode A, Weckesser M, et al. Radioligand therapy with 177Lu-PSMA-617 as A novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41(7):522–8.
Rahbar K, Schmidt M, Heinzel A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016;57(9):1334–8.
Yadav MP, Ballal S, Tripathi M, et al. (177)Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44(1):81–91.
Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41(7):1280–92.
Calopedos RJS, Chalasani V, Asher R, Emmett L, Woo HH. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20(3):352–60.
Scarpa L, Buxbaum S, Kendler D, et al. The (68)Ga/(177)Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: correlation of SUVmax values and absorbed dose estimates. Eur J Nucl Med Mol Imaging. 2017;44(5):788–800.
Ferdinandus J, Eppard E, Gaertner FC, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J Nucl Med. 2017;58(2):312–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Ladurner, W. Horninger, and J. Bektic declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ladurner, M., Horninger, W. & Bektic, J. Lutetium-PSMA therapy—a new therapeutic option in metastatic castration-resistant prostate cancer?. memo 11, 301–304 (2018). https://doi.org/10.1007/s12254-018-0452-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-018-0452-7