Abstract
Acetyl-L-carnitine (ALC) has gained clinical interest for its analgesic effect in different forms of neuropathies associated with chronic pain, such as diabetic and HIV-related peripheral neuropathies. The antinociceptive effect of ALC has been confirmed in several experimental models of neuropathic pain, including streptozotocin- and chemotherapy-induced neuropathy, and the sciatic nerve chronic constriction injury model. In these models, prophylactic administration of ALC has proven to be effective in preventing the development of neuropathic pain. In addition, ALC is known to produce a strong antinociceptive effect when given after neuropathic pain has been established. ALC can also improve the function of peripheral nerves by increasing nerve conduction velocity, reducing sensory neuronal loss, and promoting nerve regeneration.
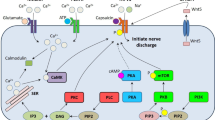
Analgesia requires repeated administrations of ALC, suggesting that the drug regulates neuroplasticity across the pain neuraxis. Recent evidence indicates that ALC regulates processes that go beyond its classical role in energy metabolism. These processes involve the activation of muscarinic cholinergic receptors in the forebrain, and an increased expression of type-2 metabotropic glutamate (mGlu2) receptors in dorsal root ganglia neurons. Induction of mGlu2 receptors is mediated by acetylation mechanisms that involve transcription factors of the nuclear factor (NF)-κB family.




Similar content being viewed by others
References
Bremer J. Carnitine: metabolism and functions. Physiol Rev 1983; 63(4): 1420–80
Farrell S, Vogel J, Bieber LL. Entry of acetyl-L-carnitine into biosynthetic pathways. Biochim Biophys Acta 1986; 876(1): 175–7
Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell 2003; 112(1): 113–22
Kido Y, Tamai I, Ohnari A, et al. Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and acetyl-L-carnitine across the blood-brain barrier. J Neurochem 2001; 79(5): 959–69
Inano A, Sai Y, Nikaido H, et al. Acetyl-L-carnitine permeability across the blood-brain barrier and involvement of carnitine transporter OCTN2. Biopharm Drug Dispos 2003; 24(8): 357–65
Dolezal V, Tucek S. Utilization of citrate, acetylcarnitine, acetate, pyruvate and glucose for the synthesis of acetylcholine in rat brain slices. J Neurochem 1981; 36(4): 1323–30
Chiechio S, Copani A, Nicoletti F, et al. L-Acetylcarnitine: a proposed therapeutic agent for painful peripheral neuropathies. Curr Neuropharmacol 2006; 4(3): 233–7
Hart AM, Wilson AD, Montovani C, et al. Acetyl-1-carnitine: a pathogenesis based treatment for HIV-associated antiretroviral toxic neuropathy. AIDS 2004; 18(11): 1549–60
Scarpini E, Sacilotto G, Baron P, et al. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst 1997; 2(3): 250–2
De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy: a long-term, randomised, double-blind, placebo-controlled study. Drugs R D 2002; 3(4): 223–31
Sima AA, Calvani M, Mehra M, et al. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005 Jan; 28(1): 89–94
Quatraro A, Roca P, Donzella C, et al. Acetyl-L-carnitine for symptomatic diabetic neuropathy. Diabetologia 1995; 38(1): 123
Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 9(2): 153–61
Moore RD, Wong WM, Keruly JC, et al. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS 2000; 14(3): 273–8
Moyle G. Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clin Therapeut 2000; 22(8): 911–36; discussion 898
Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf 1998; 19(6): 481–94
Famularo G, Moretti S, Marcellini S, et al. Acetyl-carnitine deficiency in AIDS patients with neurotoxicity on treatment with antiretroviral nucleoside analogues. AIDS 1997; 11(2): 185–90
Maestri A, De Pasquale Ceratti A, Cundari S, et al. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori 2005; 91(2): 135–8
Chiechio S, Caricasole A, Barletta E, et al. L-Acetylcarnitine induces analgesia by selectively up-regulating mGlu2 metabotropic glutamate receptors. Mol Pharmacol 2002; 61(5): 989–96
Flatters SJ, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett 2006; 397(3): 219–23
Galeotti N, Bartolini A, Calvani M, et al. Acetyl-L-carnitine requires phospholipase C-IP3 pathway activation to induce antinociception. Neuropharmacology 2004; 47(2): 286–94
Ghelardini C, Galeotti N, Calvani M, et al. Acetyl-l-carnitine induces muscarinic antinocieption in mice and rats. Neuropharmacology 2002; 43(7): 1180–7
Ghirardi O, Vertechy M, Vesci L, et al. Chemotherapy-induced allodinia: neuroprotective effect of acetyl-L-carnitine. In Vivo 2005; 19(3): 631–7
Chiechio S, Copani A, De Petris L, et al. Transcriptional regulation of metabotropic glutamate receptor 2/3 expression by the NF-kappaB pathway in primary dorsal root ganglia neurons: a possible mechanism for the analgesic effect of L-acetylcarnitine. Molecular Pain 2006; 2: 20
Chiechio S, Copani A, Melchiorri D, et al. Metabotropic receptors as targets for drugs of potential use in the treatment of neuropathic pain. J Endocrinol Invest 2004; 27(6 Suppl.): 171–6
Angelucci L, Ramacci MT, Taglialatela G, et al. Nerve growth factor binding in aged rat central nervous system: effect of acetyl-L-carnitine. J Neurosci Res 1988; 20(4): 491–6
Nakamura J, Koh N, Sakakibara F, et al. Polyol pathway hyperactivity is closely related to carnitine deficiency in the pathogenesis of diabetic neuropathy of streptozotocin-diabetic rats. J Pharmacol Exp Ther 1998; 287(3): 897–902
Lowitt S, Malone JI, Salem AF, et al. Acetyl-L-carnitine corrects the altered peripheral nerve function of experimental diabetes. Metabolism 1995; 44(5): 677–80
Sima AA, Ristic H, Merry A, et al. Primary preventive and secondary interventionary effects of acetyl-L-carnitine on diabetic neuropathy in the bio-breeding Worcester rat. J Clin Invest 1996; 97(8): 1900–7
McKay Hart A, Wiberg M, Terenghi G. Pharmacological enhancement of peripheral nerve regeneration in the rat by systemic acetyl-L-carnitine treatment. Neurosci Lett 2002; 334(3): 181–5
Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288(5472): 1765–9
Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33(1): 87–107
Pisano C, Pratesi G, Laccabue D, et al. Paclitaxel and Cisplatin-induced neurotoxicity: a protective role of acetyl-L-carnitine. Clin Cancer Res 2003; 9(15): 5756–67
Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 2006; 122(3): 245–57
Ghirardi O, Lo Giudice P, Pisano C, et al. Acetyl-L-carnitine prevents and reverts experimental chronic neurotoxicity induced by oxaliplatin, without altering its antitumor properties. Anticancer Res 2005; 25(4): 2681–7
Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer 2004; 12(9): 619–25
Finsterer J. Mitochondrial neuropathy. Clin Neurol Neurosurg 2005; 107(3): 181–6
Ghelardini C, Galeotti N, Bartolini A. Loss of muscarinic antinociception by antisense inhibition of M(1) receptors. Br J Pharmacol 2000; 129(8): 1633–40
Ghelardini C, Bartolini A, Galeotti N, et al. S-(−)-ET 126: a potent and selective M1 antagonist in vitro and in vivo. Life Sci 1996; 58(12): 991–1000
Iwamoto ET, Marion L. Characterization of the antinociception produced by intrathecally administered muscarinic agonists in rats. J Pharmacol Exp Ther 1993; 266(1): 329–38
Li DP, Chen SR, Pan YZ, et al. Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol 2002; 543(Pt 3): 807–18
Zhang HM, Chen SR, Pan HL. Regulation of glutamate release from primary afferents and interneurons in the spinal cord by muscarinic receptor subtypes. J Neurophysiol 2007; 97(1): 102–9
Dussor GO, Helesic G, Hargreaves KM, et al. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of the primary sensory neuron. Pain 2004; 107(1–2): 22–32
Bartolini A, Ghelardini C, Fantetti L, et al. Role of muscarinic receptor subtypes in central antinociception. Br J Pharmacol 1992; 105(1): 77–82
Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth Analg 1997; 85(4): 847–53
Ghelardini C, Galeotti N, Lelli C, et al. M1 receptor activation is a requirement for arecoline analgesia. Farmaco 2001; 56(5–7): 383–5
Galeotti N, Bartolini A, Ghelardini C. The phospholipase C-IP3 pathway is involved in muscarinic antinociception. Neuropsychopharmacol 2003; 28(5): 888–97
De Blasi A, Conn PJ, Pin J, et al. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci 2001; 22(3): 114–20
Varney MA, Gereau RW. Metabotropic glutamate receptor involvement in models of acute and persistent pain: prospects for the development of novel analgesics. Curr Drug Targets 2002; 1(3): 283–96
Kim SJ, Calejesan AA, Zhuo M. Activation of brainstem metabotropic glutamate receptors inhibits spinal nociception in adult rats. Pharmacol Biochem Behav 2002; 73(2): 429–37
Simmons RM, Webster AA, Kalra AB, et al. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav 2002; 73(2): 419–27
Sharpe EF, Kingston AE, Lodge D, et al. Systemic pre-treatment with a group II mGlu agonist, LY379268, reduces hyperalgesia in vivo. Br J Pharmacol 2002; 135(5): 1255–62
Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol 2006; 17(5): 592–604
Li W, Neugebauer V. Differential changes of group II and group III mGluR function in central amygdala neurons in a model of arthritic pain. J Neurophysiol 2006; 96(4): 1803–15
Jones CK, Eberle EL, Peters SC, et al. Analgesic effects of the selective group II (mGlu2/3) metabotropic glutamate receptoragonists LY379268 and LY389795 in persistent and inflammatory pain models after acute and repeated dosing.Neuropharmacol 2005; 49Suppl. 1: 206–18
Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors mediate endogenous anti-allodynia in inflammation. Pain 2003; 106(3): 411–7
Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors (mGluR2/3) regulate prostaglandin E2-mediated sensitization of capsaicin responses and thermal nociception. J Neurosci 2002; 22(15): 6388–93
Valerio A, Paterlini M, Boifava M, et al. Metabotropic glutamate receptor mRNA expression in rat spinal cord. Neuroreport 1997; 8(12): 2695–9
Berthele A, Boxall SJ, Urban A, et al. Distribution and developmental changes in metabotropic glutamate receptor messenger RNA expression in the rat lumbar spinal cord. Brain Res 1999; 112(1): 39–53
Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol 1999; 410(4): 627–42
Azkue JJ, Mateos JM, Elezgarai I, et al. The metabotropic glutamate receptor subtype mGluR 2/3 is located at extrasynaptic loci in rat spinal dorsal horn synapses. Neurosci Lett 2000; 287(3): 236–8
Gerber G, Zhong J, Youn D, et al. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience 2000; 100(2): 393–406
Das C, Kundu TK. Transcriptional regulation by the acetylation of nonhistone proteins in humans: a new target for therapeutics. IUBMB Life 2005; 57(3): 137–49
Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Reports 2003; 4(10): 944–7
Chen L, Fischle W, Verdin E, et al. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 2001; 293(5535): 1653–7
Furia B, Deng L, Wu K, et al. Enhancement of nuclear factor-kappa B acetylation by coactivator p300 and HIV-1 Tat proteins. J Biol Chem 2002; 277(7): 4973–80
Nickols JC, Valentine W, Kanwal S, et al. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci 2003; 6(2): 161–7
Acknowledgements
Experiments on the regulation of mGlu2 receptor expression by ALC were supported by the McDonnell Center for Molecular and Cellular Neurobiology and by the National Institutes of Health.
Conflict of interest: S. Chiechio currently has a fellowship from Sigma-Tau, which produces and sells carnitine. Nevertheless, the author did not receive any financial support or benefit for writing this paper. The authors have no other potential conflicts of interest that are directly relevant to this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiechio, S., Copani, A., Gereau, R.W. et al. Acetyl-L-Carnitine in Neuropathic Pain. CNS Drugs 21 (Suppl 1), 31–38 (2007). https://doi.org/10.2165/00023210-200721001-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200721001-00005