Abstract
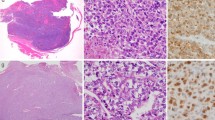
Meningeal solitary fibrous tumor (SFT) and hemangiopericytoma (HPC) were categorized as the same entity in the World Health Organization (WHO) 2016 classification of tumors of the central nervous system (CNS). Although NAB2-STAT6 fusion protein can be used to distinguish most of SFT/HPC from the other sarcomas, additional biomarkers were requested to separate meningeal SFT/HPC from meningioma and the molecular pathological difference between meningeal SFT/HPC and extra-CNS SFT/HPC still remains unclear. In this study, we evaluated the expression of TTF-1 in 67 meningeal SFT/HPC, 62 extra-CNS SFT/HPC and 201 meningiomas samples with immunohistochemistry (IHC) assays. The results showed that TTF-1 was detected in 23 of 67 (34.3%) meningeal SFT/HPC, 3 retroperitoneum SFT/HPC and none of meningiomas. Meanwhile, the copy number variation and mRNA expression of TTF-1 were measured by real-time quantitative PCR (qPCR) in meningeal SFT/HPC. These results demonstrated that TTF-1 protein expression level was significantly correlated with its transcription level, but independently related to the gene copy number variant. In conclusion, our study suggested that a large proportion of meningeal SFT/HPC was positive to TTF-1, while very few extra CNS SFT/HPC cases and no meningiomas were stained. So TTF-1 has value as an auxiliary diagnostic marker for meningeal SFT/HPC.





Similar content being viewed by others
References
Chmielecki J, Crago AM, Rosenberg M et al (2013) Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in Solitary fibrous tumors. Nat Genet 45:131–132
Robinson DR, Wu YM, Kalyana-Sundaram S et al (2013) Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 45:180–185
WHO Classification of Tumors Editorial Board (2020) WHO classification of tumours of soft tissue and bone, 5th edn. IARC Press, Lyon
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Ishizawa K, Tsukamoto Y, Ikeda S et al (2016) ‘Papillary’ solitary fibrous tumor/hemangiopericytoma with nuclear STAT6 expression and NAB2-STAT6 fusion. Brain Tumor Pathol 33:151–156
Schweizer L, Koelsche C, Sahm F et al (2013) Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2/STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol 125:651–658
Ouladan S, Trautmann M, Orouji E et al (2015) Differential diagnosis of solitary fibrous tumors: a study of 454 soft tissue tumors indicating the diagnostic value of nuclear STAT6 relocation and ALDH1 expression combined with in situ proximity ligation assay. Int J Oncol 46:2595–2605
Doyle LA, Vivero M, Fletcher CD et al (2014) Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 27:390–395
Mocagno N, Figarella-Branger D, Mokthari K et al (2016) Differential diagnosis of meningeal SFT-HPC and meningioma, which immunohistochemical markers should be used? Am J Surg Pathol 40:270–278
Pelizzoli R, Tacchetti C, Luzzi P et al (2008) TTF-1/NKX2.1 up-regulates the in vivo transcription of nestin. Int J Dev Biol 52:55–62
Hang JF, Hsu CY, Lin SC et al (2017) Thyroid transcription factor-1 distinguishes subependymal giant cell astrocytoma from its mimics and supports its cell origin from the progenitor cells in the medial ganglionic eminence. Mod Pathol 30:318–328
Ordóñez NG (2012) Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol 20:429–444
Kristensen MH, Nielsen S, Vyberg M (2011) Thyroid transcription factor-1 in primary CNS tumors. Appl Immunohistochem Mol Morphol 19:437–443
Bielle F, Villa C, Giry M et al (2015) Chordoid gliomas of the third ventricle share TTF-1 expression with organum vasculosum of the lamina terminalis. Am J Surg Pathol 39:948–956
Wang DZ, Liu P, Yao L et al (2018) Aberrant expression of thyroid transcription factor-1 in schwannomas. Hum Pathol 71:84–90
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2− ΔΔCt method. Methods 25(4):402–408
Wang R, Carter J, Lench N (2013) Evaluation of real-time quantitative PCR as a standard cytogenetic diagnostic tool for confirmation of microarray (aCGH) results and determination of inheritance. Genet Test Mol Biomark 17:821–825
Ramamoorthy A, Flockhart DA, Hosono N et al (2010) Differential quantification of CYP2D6 gene copy number by four different quantitative real-time PCR assays. Pharmacogenet Genom 20:451–454
Barthelmeß S, Geddert H, Boltze C et al (2014) Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2/STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol 184:1209–1218
Nakada S, Minato H, Nojima T (2016) Clinicopathological differences between variants of the NAB2- STAT6 fusion gene in solitary fibrous tumors of the meninges and extra-central nervous system. Brain Tumor Pathol 33:169–174
McDaniel AS, Palanisamy N, Smith SC et al (2016) A subset of solitary fibrous tumors express nuclear PAX8 and PAX2: a potential diagnostic pitfall. Histol Histopathol 31:223–230
Compérat E, Zhang F, Perrotin C et al (2005) Variable sensitivity and specificity of TTF-1 antibodies in lung metastatic adenocarcinoma of colorectal origin. Mod Pathol 18:1371–1376
Bertucci F, Bouvier-Labit C, Finetti P et al (2013) Gene expression profiling of solitary fibrous tumors. PLoS ONE 8:e64497
Benlhabib H, Mendelson CR (2011) Epigenetic regulation of surfactant protein A gene (SP-A) expression in fetal lung reveals a critical role for Suv39h methyltransferases during development and hypoxia. Mol Cell Biol 31:1949–1958
Kondo T, Nakazawa T, Ma D et al (2009) Epigenetic silencing of TTF-1/NKX2-1 through DNA hypermethylation and histone H3 modulation in thyroid carcinomas. Lab Invest 89:791–799
Roato I, Ferracini R (2018) Cancer stem cells, bone and tumor microenvironment: key players in bone metastases. Cancers (Basel) 10(2):56
Easwaran H, Tsai HC, Baylin SB (2014) Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell 54:716–727
Neradil J (2015) Veselska R (2015) Nestin as a marker of cancer stem cells. Cancer Sci 106:803–811
Wang C, Qi Y, Liu R et al (2015) Immunohistochemical evaluation of stem cell markers and signal transducer and activator of transcription 6 (STAT6) in solitary fibrous tumors. Int J Clin Exp Pathol 8:10585–10594
Hamm CA, Stevens JW, Xie H et al (2010) Microenvironment alters epigenetic and gene expression profiles in swarm rat chondrosarcoma tumors. BMC Cancer 10:471
Devaud C, Westwood JA, John LB et al (2014) Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther 22:18–27
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (WK9110000042); the Natural Science Foundation of Anhui Province (1908085MH281); and the National Natural Science Foundation of China (81702954).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, H., Du, J., Li, H. et al. Aberrant expression of thyroid transcription factor-1 in meningeal solitary fibrous tumor/hemangiopericytoma. Brain Tumor Pathol 38, 122–131 (2021). https://doi.org/10.1007/s10014-021-00395-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-021-00395-1