Abstract
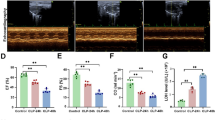
Apoptosis is an important process in a wide variety of different biological systems. In addition to caspases, recently, calpains, another family of proteases, have been found to be involved in apoptosis of many cell systems. This study is designed with the aims to evaluate the possible effect of Z-LLY-FMK (a calpain inhibitor) on intestine apoptosis after bile duct ligation in rats. Male Sprague–Dawley rats weighing 250–300 g were randomized to five groups (n = 6 in each group). Group 1 (Control: C) underwent Sham operation and were simultaneously treated with the same amount of normal saline. Group 2 (Control with DMSO: CDMSO) underwent Sham operation and were simultaneously treated with the same amount of dimethylsulfoxide (DMSO). Group 3 (Obstructive jaundice: OB) underwent common bile duct ligation without any other manipulation. Group 4 (Obstructive jaundice with Z-LLY-FMK: OBZLLY) underwent common bile duct ligation and were simultaneously treated with Z-LLY-FMK (dissolved in DMSO). Group 5 (Obstructive jaundice with ZFA-FMK: OBZFA) underwent common bile duct ligation and were simultaneously treated with ZFA-FMK (dissolved in DMSO). After 3 days, intestine tissue was harvested for apoptosis measurements. There was no significant difference between Sham operation group (C) and Sham operation with DMSO group (CDMSO) either in jejunum (P = 0.924) or in ileum (P = 0.996). When compared to Sham operation group (C), increased intestine apoptosis occurred in either jejunum (P < 0.001) or in ileum (P < 0.001) after common bile duct ligation (OB). After administration of Z-LLY-FMK (OBZLLY), the increased intestine apoptosis after common bile duct ligation (OB) was significantly diminished either in jejunum or in ileum (P < 0.001 and P < 0.001). Moreover, administration of ZFA (OBZFA) failed to show the same phenomenon in either jejunum (P = 0.993) or ileum (P = 0.485). There was a significant difference in intestine apoptosis in either jejunum (P < 0.001) or in ileum (P < 0.001) between OBZLLY group and OBZFA group. Significantly increased intestine apoptosis occurred after common bile duct ligation. The administration of Z-LLY-FMK could effectively diminish the intestine apoptosis after common bile duct ligation, whereas the administration of ZFA-FMK failed to show the same effect.


Similar content being viewed by others
References
Pitt HA, Cameron JL, Postier RG, et al. Factors affecting mortality in biliary tract surgery. Am J Surg. 1981;141:66–72. doi:10.1016/0002-9610(81)90014-3.
Su CH, Peng FK, Lui WY. Factors affecting morbidity and mortality in biliary tract surgery. World J Surg. 1992;16:536–540. doi:10.1007/BF02104465.
Deitch EA, Sitting K, Berg R, et al. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79–84.
Bossuyt HV, Desmaretz C, Gaeta GB, Wisse E. The role of bile acids in the development of endotoxemia during obstructive jaundice in the rat. J Hepatol. 1990;10:274–279.
Sheen-Chen SM, Chau P, Harris HW. Obstructive jaundice alters Kupffer cell function independent of bacterial translocation. J Surg Res. 1998;80:205–209.
Souba WW, Smith RJ, Wilmore DW. Glutamine metabolism by the intestinal tract. JPEN. 1985;9:608–616.
Kaiserlian D, Vidal K. Antigen presentation by intestinal epithelial cells. Immunol Today. 1993;14:144.
Arnaiz-Villena A, Perez-Blas M, Corell A, Lopez-Suarez JC, et al. Cell surface phenotype and ultramicroscopic analysis of purified human enterocytes: a possible antigen-presenting cell in the intestine. Tissue Antigens. 1997;50:586–592.
Marin ML, Greenstein AJ, Geller SA, et al. A freeze fracture study of Crohn’s disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78:537–547.
Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biology phenomenon with wide-ranging implication in tissue kinetics. Br J Cancer. 1972;26:239–257.
Allen RT, Hunter WJIII, Agrawae DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997;37:215–228.
Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16.
Seiger CP. Anthranoid laxative and colorectal cancer. Trends Pharmacol Sci. 1992;13:229–231.
Branconnier RJ, Branconnier ME, Walshe TM, et al. Blocking the Ca (+2) activated cytotoxic mechanisms of cholinergic neuronal death: a novel treatment strategy for Alzheimer’s disease. Psychopharmacol Bull. 1992;28:175–178.
Vanags DM, Porn-Ares MI, Coppola S, et al. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085.
Wood DE, Newcomb EW. Caspase-dependent activation of calpain during drug-induced apoptosis. J Biol Chem. 1999;274:8309–8315.
Sheen-Chen SM, Chau P, Harris HW. Obstructive jaundice alters Kupffer cell function independent of bacterial translocation. J Surg Res. 1998;80(2):205–209.
Sheen-Chen SM, Chen HS, Ho HT, Chen WJ, Sheen CC, Eng HL. Effect of bile acid replacement on endotoxin-induced tumor necrosis factor-alpha production in obstructive jaundice. World J Surg. 2002;26:448–450.
Sheen-Chen SM, Ho HT, Chen WJ, et al. Obstructive jaundice alters CD44 expression in rat small intestine. Digestion. 2002;65:112–117.
Sheen-Chen SM, Chen HS, HO HT, et al. Obstructive jaundice alters LFA-1 alpha expression in rat small intestine. Dig Dis Sci. 2003;48:1165–1170.
Sheen-Chen SM, Ho HT, et al. Obstructive jaundice alters proliferating cell nuclear antigen expression in rat small intestine. World J Surg. 2003;27:1161–1164.
Sheen-Cnen SM, Hung KS, Ho Ht, Chen WJ, Eng HL. Effect of glutamine and bile acid on hepatocyte apoptosis after bile duct ligation in the rat. World J Surg. 2004;28:457–460.
Sheen-Chen SM, et al. Altered serum transforming growth factor β-1 and monocyte chemoattractant protein-1 levels in obstructive jaundice. World J Surg. 2004;28:967–970.
Cahill CJ. Prevention of postoperative renal failure in patients with obstructive jaundice—the role of bile salts. Br J Surg. 1983;70:590–595.
Clement WDB, McCaigue M, Erwin P, et al. Biliary decompression promotes Kupffer cell recovery in obstructive jaundice. Gut. 1996;38:925–931.
Paks RW, Clement WDB, Smye MG, et al. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345–1349.
Kashio Y, Nakamura K, Abedin MJ, et al. Galectin-9 induces apoptosis through the calcium–calpain–caspase-1 pathway. J Immunol. 2003;170:3631–3636.
Bravo R, Frank R, Blundell PA, et al. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature. 1987;326:515–517.
Danova M, Riccardi A, Giordano M, et al. Cell cycle-related protein: a flow cytometric study in human tumors. Biol Cell. 1988;64:23–28.
Acknowledgments
This study was supported by grant CMRPG83028 from the Chang Gung Memorial Hospital, Chang Gung University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheen-Chen, SM., Ho, HT. & Eng, HL. Z-LLY-FMK Attenuates Intestinal Apoptosis After Bile Duct Ligation in Rats. Dig Dis Sci 54, 2357–2361 (2009). https://doi.org/10.1007/s10620-008-0652-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0652-9