Abstract
Background
Even though allergies are an important health issue, wide manufacturer-dependent differences in the detected amounts of allergen-specific IgE (sIgE) have repeatedly been found. These discrepancies hinder diagnostics and research into clinically significant cutoff points for life-threatening symptoms.
Methods
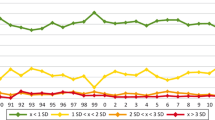
To evaluate whether the reported differences have led to changes in diagnostic testing, we analyzed data from six years of round robin testing (RRT, also known as proficiency testing) at the Institute of Standardization and Quality Control in Medical Laboratories (INSTAND e. V.) for the important allergen sources bee venom, wasp venom, and birch pollen. The results of the four main suppliers of in vitro diagnostic sIgE testing were compared in a pseudo-anonymized form using overlay images of box plot graphs for the semiquantitative data and allergen class results. Coefficients of variation (CV) were obtained to study the development of interlaboratory comparability.
Results
We found that the large differences between the manufacturer collectives remained constant between January 2010 and April 2015 without any real improvement. The CVs were good for two of the four analyzed suppliers, one was marginal and one above the quality level of 20 %.
Conclusion
The numerous publications that have found discrepancies in the sIgE results of the different suppliers did not change the status quo within the last six years. Unfortunately, this is unlikely to change until there is a characterized standard material with known values of sIgE.




Similar content being viewed by others
Abbreviations
- CCD:
-
Cross-reactive carbohydrate structure
- CV:
-
Coefficient of variation
- INSTAND:
-
Institute of Standardization and Quality Control in Medical Laboratories
- RfB:
-
Reference Institute for Bioanalytics
- RRT:
-
Round robin test
- sIgE:
-
Specific IgE
References
Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss M. The WAO White Book on Allergy (Update. 2013).
Haftenberger M, Laußmann D, Ellert U, Kalcklösch M, Langen U, Schlaud M, et al. Prävalenz von Sensibilisierungen gegen Inhalations- und Nahrungsmittelallergene: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:687–97
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63 Suppl 86:8–160
Langen U, Schmitz R, Steppuhn H. Häufigkeit allergischer Erkrankungen in Deutschland: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:698–706
Worm M, Eckermann O, Dolle S, Aberer W, Beyer K, Hawranek T et al. Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany, Austria and Switzerland. Dtsch Arztebl Int 2014;111:367–75
Schellenberg I, Göring HD, Kabrodt K. Fünf Jahre INSTAND-Ringversuche zur In-vitro-Allergiediagnostik - ein Beitrag zur Qualitätssicherung in der medizinischen Diagnostik. Allergologie 2003;26:1–14
Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol 2010;125.S284–96
Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol 2008;121:1219–24
Ollert M, Weissenbacher S, Rakoski J, Ring J. Allergen-specific IgE measured by a continuous random-access immunoanalyzer: interassay comparison and agreement with skin testing. Clin Chem 2005;51:1241–49
Li TM, Chuang T, Tse S, Hovanec-Burns D, El Shami AS. Development and validation of a third generation allergen-specific IgE assay on the continuous random access IMMULITE 2000 analyzer. Ann Clin Lab Sci 2004;34:67–74
Lee YW, Sohn JH, Lee J, Hong C, Park J. Allergen-specific IgE measurement with the IMMULITE 2000 system: intermethod comparison of detection performance for allergen-specific IgE antibodies from Korean allergic patients. Clin Chim Acta 2009;401:25–32
Koch L, Aberer W. Comparability and quality of IgE-based in vitro allergy diagnosis: 25 years of external quality assessment. Wien Klin Wochenschr 2014;126:634–41
Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol 2007;99:34–41
Kleine-Tebbe J, Matricardi PM, Hamilton RG. Allergy Work-Up Including Component-Resolved Diagnosis: How to Make Allergen-Specific Immunotherapy More Specific. Immunol Allergy Clin North Am 2016;36:191–203
Bundesärztekammer. Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen: RiLiBÄK. Dtsch Arztebl. 2014;111(38):A1583-A1618.
Schenk MF, Cordewener JHG, America AHP, Van’t Westende, Wendy P C, Smulders MJM, et al. Characterization of PR-10 genes from eight Betula species and detection of Bet v 1 isoforms in birch pollen. BMC Plant Biol 2009;9:24
Gajhede M, Osmark P, Poulsen FM, Ipsen H, Larsen JN, Joost van Neerven R J, et al. X-ray and NMR structure of Bet v 1, the origin of birch pollen allergy. Nat Struct Biol 1996;3:1040-4
Holm J, Gajhede M, Ferreras M, Henriksen A, Ipsen H, Larsen JN, et al. Allergy vaccine engineering: epitope modulation of recombinant Bet v 1 reduces IgE binding but retains protein folding pattern for induction of protective blocking-antibody responses. J Immunol} 2004;173:5258–67
Markovic-Housley Z, Degano M, Lamba D, Roepenack-Lahaye E von, Clemens S, Susani M, et al. Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. J Mol Biol 2003;325:123–33
Movérare R, Westritschnig K, Svensson M, Hayek B, Bende M, Pauli G, et al. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol 2002;128:325–35
Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JNG. Diagnosis of Hymenoptera venom allergy. Allergy 2005;60:1339–49
Blum S, Gunzinger A, Muller UR, Helbling A. Influence of total and specific IgE, serum tryptase, and age on severity of allergic reactions to Hymenoptera stings. Allergy 2011;66:222–8
Golden DBK. Insect sting anaphylaxis. Immunol Allergy Clin North Am 2007;27:261-72, vii
Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol 2001;107:897–901
Vos B, Kohler J, Muller S, Stretz E, Rueff F, Jakob T. Spiking venom with rVes v 5 improves sensitivity of IgE detection in patients with allergy to Vespula venom. J Allergy Clin Immunol 2013;131:1225–7, 1227.e1
Blank S, Seismann H, Michel Y, McIntyre M, Cifuentes L, Braren I, et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy 2011;66:1322–9
Sturm GJ, Jin C, Kranzelbinder B, Hemmer W, Sturm EM, Griesbacher A, et al. Inconsistent results of diagnostic tools hamper the differentiation between bee and vespid venom allergy. PLoS One 2011;6:e20842
Hemmer W. Kreuzreaktionen zwischen Hymenopterengiftallergien. Allergo Journal 2009;18:359–72
Jappe U, Raulf-Heimsoth M, Hoffmann M, Burow G, Hubsch-Muller C, Enk A. In vitro hymenoptera venom allergy diagnosis: improved by screening for cross-reactive carbohydrate determinants and reciprocal inhibition. Allergy 2006;61:1220–9
van Ree R. Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int Arch Allergy Immunol 2002;129:189–97
Mahler V, Gutgesell C, Valenta R, Fuchs T. Natural rubber latex and hymenoptera venoms share Immunoglobin E-epitopes accounting for cross-reactive carbohydrate determinants. Clin Exp Allergy 2006;36:1446–56
Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol 2007;142:99–115
Mittermann I, Zidarn M, Silar M, Markovic-Housley Z, Aberer W, Korosec P, et al. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J Allergy Clin Immunol 2010;125:1300–07.e3
Tretter V, Altmann F, Kubelka V, Marz L, Becker WM. Fucose alpha 1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int Arch Allergy Immunol 1993;102:259–66
Hemmer W, Focke M, Kolarich D, Dalik I, Gotz M, Jarisch R. Identification by immunoblot of venom glycoproteins displaying immunoglobulin E-binding N-glycans as cross-reactive allergens in honeybee and yellow jacket venom. Clin Exp Allergy 2004;34:460–9
Muller UR, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy 2009;64:543–8
Wojtalewicz N, Goseberg S, Kabrodt K, Schellenberg I. 6 years of INSTAND e.V. sIgE round robin tests: An evaluation of in vitro allergy diagnostics for food allergens. GMS Zeitschrift zur Förderung der Qualitätssicherung in medizinischen Laboratorien 2016 (in press).
Wojtalewicz N, Goseberg S, Kabrodt K, Schellenberg I. 6 years of INSTAND e.V. sIgE round robin tests: An evaluation of in vitro allergy diagnostics for inhaled allergens. GMS Zeitschrift zur Förderung der Qualitätssicherung in medizinischen Laboratorien 2016 (in press).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ contributions
Wojtalewicz N. performed the statistical analysis, generated the figures, and wrote the paper. Goseberg S. collected and pooled the INSTAND e. V. data. Kabrodt K. corrected the paper and Schellenberg I. is the corresponding author as well as the EQA expert for in vitro allergy round robin tests. He is also vice president of INSTAND e. V.
Acknowledgements
We would like to thank King for proofreading the manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest.
Cite this as
Wojtalewicz N, Goseberg S, Kabrodt K, Schellenberg I. Six years of INSTAND e. V. sIgE proficiency testing: an evaluation of in vitro allergy diagnostics. Allergo J Int 2017; 26: 43–52
DOI: 10.1007/s40629-016-0005-8
Rights and permissions
About this article
Cite this article
Wojtalewicz, N., Goseberg, S., Kabrodt, K. et al. Six years of INSTAND e. V. sIgE proficiency testing: an evaluation of in vitro allergy diagnostics. Allergo J 26, 20–29 (2017). https://doi.org/10.1007/s15007-017-1297-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15007-017-1297-9