Abstract
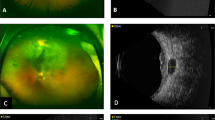
To evaluate uveal melanoma cell activity and pathologic features after stereotactic CyberKnife radiosurgery in specimens from five patients. Specimens from five patients treated by CyberKnife radiosurgery in three fractions were included in this study. Because of persistent retinal detachment in 3 patients, tumour endoresection was performed at four, seven and ten month after CyberKnife radiosurgery. At nine and twelve months after treatment, enucleation of the eye globe was performed in 2 patients because of secondary tumour bleeding and missing regression. After histomorphological analysis and determination of Ki67-proliferation index, DNA cytophotometry, fluorescence in-situ hybridization evaluation for chromosome 3 loss, GNA11and GNAQ mutation analysis were performed. Four of the five tumours included in this study showed variable radiation-induced morphologic changes in the form of enlargement of cells and nuclei, cytoplasmic vacuolisation and nuclear fragmentation. The DNA content of a large fraction of tumour cells was hypoploid. On the other hand, single strikingly hyperchromatic melanoma cells showed marked aneuploidy. The proliferation fraction in the three endoresected tumours was very low (<1%), but it was elevated in the enucleation cases. Monosomy 3 was detected in two of the endoresection cases, but none of the enucleation cases. None of the patients experienced a local tumour recurrence, but two of the patients developed liver metastasis. Many melanoma cells seemed to be vital within the first 6 months after CyberKnife radiosurgery, but obvious radiation-induced morphologic changes, including tumour necrosis, hypoploid DNA content plus low Ki-67 index could indicate sublethal cell damage.



Similar content being viewed by others
References
Eagle CR (2011) Eye pathology. An atlas and text. Lippincott Williams & Wilkins, Philadelphia, pp 177–206
Font RL, Croxatto JO, Rao NA (2006) AFIP atlas of tumor pathology. Tumors of the eye and ocular adnexa. Armed Forces Institute of Pathology, Washington DC
Scotto J, Fraumeni JF, Lee JA (1976) Melanomas of the eye and other noncutaneous sites: epidemiologic aspects. J Natl Cancer Inst 56:489–491
Bergman L, Seregard S, Nilsson B, Lundell G, Ringborg U, Ragnarsson-Olding B (2002) Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 43:2579–2583
Singh AD, Topham A (2003) Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 110:956–961
Damato B (2012) Progress in the management of patients with uveal melanoma. The 2012 Ashton lecture. Eye (Lond) 2012 Sep; 26(9):1157-72. https://doi.org/10.1038/eye.2012.126
American Brachytherapy Society - Ophthalmic Oncology Task Force (2014) The American brachytherapy society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 13:1–14. https://doi.org/10.1016/j.brachy.2013.11.008
Damato B (1997) Adjunctive plaque radiotherapy after local resection of uveal melanoma. Front Radiat Ther Oncol 30:123–132
Bechrakis NE, Bornfeld N, Zoller I, Foerster MH (2002) Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 109:1855–1861
Zorlu F, Selek U, Kiratli H (2009) Initial results of fractionated CyberKnife radiosurgery for uveal melanoma. J Neuro-Oncol 94:111–117. https://doi.org/10.1007/s11060-009-9811-x
Wackernagel W, Holl E, Tarmann L, Mayer C, Avian A, Schneider M, Kapp KS, Langmann G (2014) Local tumour control and gamma-knife radiosurgery of choroidal melanomas. Br J Ophthalmol 98:218–223. https://doi.org/10.1136/bjophthalmol-2013-304031
Schirmer CM, Chan M, Mignano J, Duker J, Melhus CS, Williams LB, Wu JK, Yao KC (2009) Dose de-escalation with gamma knife radiosurgery in the treatment of choroidal melanoma. Int J Radiat Oncol Biol Phys 75:170–176. https://doi.org/10.1016/j.ijrobp.2008.10.077
Sarici AM, Pazarli H (2013) Gamma-knife-based stereotactic radiosurgery for medium- and large-sized posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol 251:285–294. https://doi.org/10.1007/s00417-012-2144-z
Damato B, Patel I, Campbell IR, Mayles HM, Errington RD (2005) Local tumor control after (106)Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys 63:385–391
Russo A, Laguardia M, Damato B (2012) Eccentric ruthenium plaque radiotherapy of posterior choroidal melanoma. Graefes Arch Clin Exp Ophthalmol 250(10):1533–1540. https://doi.org/10.1007/s00417-012-1962-3
Stoffelns BM, Kutzner J, Jochem T (2002) Retrospective analysis of ruthenium-106 brachytherapy for small and medium-sized malignant melanoma of the posterior choroid. Klin Monatsbl Augenheilkd 219(4):216–220. https://doi.org/10.1055/s-2002-30654
Shields CL, Cater J, Shields JA, Chao A, Krema H, Materin M, Brady LW (2002) Combined plaque radiotherapy and transpupillary thermotherapy for choroidal melanoma: tumor control and treatment complications in 270 consecutive patients. Arch Ophthalmol 120:933–940
Klaus H, Lommatzsch PK, Fuchs U (1991) Histopathology studies in human malignant melanomas of the choroid after unsuccessful treatment with106Ru/106Rh ophthalmic applicators. Graefes Arch Clin Exp Ophthalmol 229:480–486
Heindl LM, Lotter M, Strnad V, Sauer R, Naumann GO, Knorr HL (2007) High-dose 106Ruthenium plaque brachytherapy for posterior uveal melanoma. A clinico-pathologic study. Ophthalmologe 104:149–157. https://doi.org/10.1007/s00347-006-1451-3
no authors listed (1998) Histopathologic characteristics of uveal melanomas in eyes enucleated from the collaborative ocular melanoma study. COMS report no. 6. Am J Ophthalmol 125:745–766
Avery RB, Diener-West M, Reynolds SM, Grossniklaus HE, Green WR, Albert DM (2008) Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch Ophthalmol 126:207–212. https://doi.org/10.1001/archopthalmol.2007.50
Shields CL, Shields JA, Karlsson U, Menduke H, Brady LW (1990) Enucleation after plaque radiotherapy for posterior uveal melanoma. Histopathologic findings. Ophthalmology 97:1665–1670
Brierley JD, Gospodarowicz MK, Wittekind C (eds) (2017) 8th edition of the Union for International Cancer Control / American Joint Committee on Cancer TNM classification. Wiley, Oxford
McLean IW, Foster WD, Zimmerman LE, Gamel JW (1983) Modifications of Callender’s classification of uveal melanoma at the armed forces Institute of Pathology. Am J Ophthalmol 96:502–209
Gouvêa AF, Santos Silva AR, Speight PM, Hunter K, Carlos R, Vargas PA, de Almeida OP, Lopes MA (2013) High incidence of DNA ploidy abnormalities and increased Mcm2 expression may predict malignant change in oral proliferative verrucous leukoplakia. Histopathology 62:551–562. https://doi.org/10.1111/his.12036
Schilling H, Sehu KW, Lee WR (1997) A histologic study (including DNA quantification and Ki-67 labeling index) in uveal melanomas after brachytherapy with ruthenium plaques. Invest Ophthalmol Vis Sci 38:2081–2092
Van den Bosch T, van Beek JG, Vaarwater J, Verdijk RM, Naus NC, Paridaens D, de Klein A, Kiliç E (2012) Higher percentage of FISH-determined monosomy 3 and 8q amplification in uveal melanoma cells relate to poor patient prognosis. Invest Ophthalmol Vis Sci 53:2668–2674. https://doi.org/10.1167/iovs.11-8697
Schneider B, Riedel K, Zhivov A, Huehns M, Zettl H, Guthoff RF, Jünemann A, Erbersdobler A, Zimpfer A (2017) Frequent and Yet Unreported GNAQ and GNA11 Mutations are found in Uveal Melanomas. Pathol Oncol Res. https://doi.org/10.1007/s12253-017-0371-7
Rand RW, Khonsary A, Brown WJ, Winter J, Snow HD (1987) Leksell stereotactic radiosurgery in the treatment of eye melanoma. Neurol Res 9:142–146
Kang DW, Lee SC, Park YG, Chang JH (2012) Long-term results of gamma knife surgery for uveal melanomas. J Neurosurg 117:108–114
Crawford JB, Char DH (1987) Histopathology of uveal melanomas treated with charged particle radiation. Ophthalmol 94:639–643
Gragoudas ES, Egan KM, Saornil MA, Walsh SM, Albert DM, Seddon JM (1993) The time course of irradiation changes in proton beam-treated uveal melanomas. Ophthalmology 100:1555–1559
Singh AD, Eagle RC Jr, Shields CL, Shields JA (2003) Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol 121:397–400
Fernandes BF, Weisbrod D, Yücel YH, Follwell M, Krema H, Heydarian M, Xu W, Payne D, McGowan H, Simpson ER, Laperriere N, Sahgal A (2011) Neovascular glaucoma after stereotactic radiotherapy for juxtapapillary choroidal melanoma: histopathologic and dosimetric findings. Int J Radiat Oncol Biol Phys 80:377–384. https://doi.org/10.1016/j.ijrobp.2010.04.073
Song WK, Yang WI, Byeon SH, Koh HJ, Kwon OW, Lee SC (2010) Clinicopathologic report of uveal melanoma with persistent exudative retinal detachment after gamma knife radiosurgery. Ophthalmologica 224:16–21. https://doi.org/10.1159/000233231
Chappell MC, Char DH, Cole TB, Harbour JW, Mishra K, Weinberg VK, Phillips TL (2012) Uveal melanoma: molecular pattern, clinical features, and radiation response. Am J Ophthalmol 154:227–232. https://doi.org/10.1016/j.ajo.2012.02.022
Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jöckel KH, Becher R (1996) Prognostic implications of monosomy 3 in uveal melanoma. Lancet 347:1222–1225
Coupland SE, Lake SL, Zeschnigk M, Damato BE (2013) Molecular pathology of uveal melanoma. Eye 27:230–242. https://doi.org/10.1038/eye.2012.255
Seregard S, Lundell G, Lax I, af Trampe E, Kock E (1997) Tumor cell proliferation after failed ruthenium plaque radiotherapy for posterior uveal melanoma. Acta Ophthalmol Scand 75:148–154
Chiquet C, Grange JD, Ayzac L, Chauvel P, Patricot LM, Devouassoux-Shisheboran M (2000) Effects of proton beam irradiation on uveal melanomas: a comparative study of Ki-67 expression in irradiated versus non-irradiated melanomas. Br J Ophthalmol 84:98–102
Pe'er J, Stefani FH, Seregard S, Kivela T, Lommatzsch P, Prause JU, Sobottka B, Damato B, Chowers I (2001) Cell proliferation activity in posterior uveal melanoma after Ru-106 brachytherapy: an EORTC ocular oncology group study. Br J Ophthalmol 85:1208–1212
Acknowledgements
We thank Mrs. Koelbel, Mrs. Westphal, Mrs. Stegemann and Mrs. Schmidtgen for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zimpfer, A., Schneider, B., Blanck, O. et al. Pathologic Features of Tumor Activity and Stability in Uveal Melanoma Specimens after Fractionated CyberKnife Radiosurgery. Pathol. Oncol. Res. 25, 731–740 (2019). https://doi.org/10.1007/s12253-018-00565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-00565-1