Abstract
Aim
The potential of intensity-modulated radiation therapy (IMRT) as opposed to three-dimensional conformal radiotherapy (3D-CRT) is analyzed for two different concepts of fluorodeoxyglucose positron emission tomography (FDG PET)-based target volume delineation in locally advanced non-small cell lung cancer (LA-NSCLC): involved-field radiotherapy (IF-RT) vs. elective nodal irradiation (ENI).
Methods
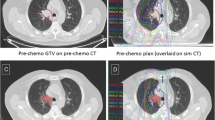
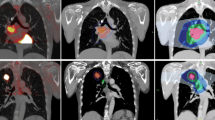
Treatment planning was performed for 41 patients with LA-NSCLC, using four different planning approaches (3D-CRT-IF, 3D-CRT-ENI, IMRT-IF, IMRT-ENI). ENI included a boost irradiation after 50 Gy. For each plan, maximum dose escalation was calculated based on prespecified normal tissue constraints. The maximum prescription dose (PD), tumor control probability (TCP), conformal indices (CI), and normal tissue complication probabilities (NTCP) were analyzed.
Results
IMRT resulted in statistically significant higher prescription doses for both target volume concepts as compared with 3D-CRT (ENI: 68.4 vs. 60.9 Gy, p < 0.001; IF: 74.3 vs. 70.1 Gy, p < 0.03). With IMRT-IF, a PD of at least 66 Gy was achieved for 95 % of all plans. For IF as compared with ENI, there was a considerable theoretical increase in TCP (IMRT: 27.3 vs. 17.7 %, p < 0.00001; 3D-CRT: 20.2 vs. 9.9 %, p < 0.00001). The esophageal NTCP showed a particularly good sparing with IMRT vs. 3D-CRT (ENI: 12.3 vs. 30.9 % p < 0.0001; IF: 15.9 vs. 24.1 %; p < 0.001).
Conclusion
The IMRT technique and IF target volume delineation allow a significant dose escalation and an increase in TCP. IMRT results in an improved sparing of OARs as compared with 3D-CRT at equivalent dose levels.
Zusammenfassung
Zielsetzung
Das Potenzial der intensitätsmodulierten Strahlentherapie (IMRT) soll im Rahmen der FDG-PET basierten Bestrahlungsplanung des lokal fortgeschrittenen nichtkleinzelligen Bronchialkarzinoms (LA-NSCLC) für 2 Zielvolumenansätze (Involved-Field-Bestrahlung, IF) sowie elektive Nodalbestrahlung (ENI) geprüft und mit der 3-D-konformalen Strahlentherapie (3-D-CRT) als Referenz verglichen werden.
Material und Methoden
Die Bestrahlungsplanung erfolgte an CT-Datensätzen von 41 Patienten mit LA-NSCLC in 4 Ansätzen (3-D-CRT-IF, 3-D-CRT-ENI, IMRT-IF, IMRT-ENI) jeweils mit 2 Gy Einzeldosis. ENI beinhaltete einen zusätzlichen Boost nach 50 Gy. Für jeden Plan wurde die maximal mögliche Dosiseskalation nach vordefinierten Grenzwerten für Normalgewebe vorgenommen.
Es wurden die maximal erreichbare Dosis, die Tumorkontrollwahrscheinlichkeit (TCP), der Konformitätsindex (CI) und Normalgewebsrisiken nach dem NTCP-Modell („normal tissue complication probabilities“) analysiert.
Ergebnisse
Die IMRT-Pläne resultierten in statistisch signifikant höheren Gesamtdosen für beide Zielvolumenkonzepte im Vergleich zur 3-D-CRT (ENI: 68,4 Gy vs. 60,9 Gy; p < 0,001; IF: 74,3 vs. 70,1 Gy; p < 0,03). Mittels IMRT-IF wurde eine Gesamtdosis von 66 Gy für 95 % der Pläne erreicht. Der rechnerische TCP-Anstieg mittels IF im Vergleich zu ENI war erheblich (IMRT: 27,3 vs. 17,7 %; p < 0,00001; 3-D-CRT: 20,2 vs. 9,9 %; p < 0,00001). NTCP-Werte für den Ösophagus waren mit der IMRT im Vergleich zur 3-D-CRT signifikant niedriger (ENI: 12,3 vs. 30,9 %; p < 0,0001; IF: 15,9 vs. 24,1 %; p < 0,001).
Schlussfolgerung
Mittels IMRT und IF lässt sich beim LA-NSCLC eine signifikante Dosiseskalation und ein Anstieg der TCP erreichen. Die IMRT ermöglicht außerdem eine signifikant geringere Normalgewebsbelastung im Vergleich zur 3-D-CRT bei äquivalenten Gesamtdosen.


Similar content being viewed by others
References
Harris JP, Murphy JD, Hanlon A et al (2014) A population based comparative effectiveness study of radiotherapy techniques in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 88:872–884
Shirvani SM, Jiang J, Gomez DR et al (2013) Intensity modulated radiotherapy for stage III non-small cell lung cancer in the United States: predictors of use and association with toxicities. Lung Cancer 82:252–259
Bortfeld T, Jokivarsi K, Goitein M et al (2002) Effects of intra-fraction motion on IMRT dose delivery: statistical analysis and simulation. Phys Med Biol 47:2203–2220
Schaefer M, Münter MW, Thilmann C et al (2004) Influence of intra-fractional breathing movement in step-and-shoot IMRT. Phys Med. Biol 49:175–179
Liao ZX, Komaki RR, Thames HD et al (2010) Influence of technologic advances on outcomes in patients with unresectabe, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys 76:775–781
Bradley J, Bae K, Choi N et al (2012) A phase II comparative study of gross tumor volume definition with or without PET/CT fusion in dosimetric planning for non-small-cell lung cancer (NSCLC): primary analysis of Radiation Therapy Oncology Group (RTOG) 0515. Int J Radiat Oncol Biol Phys 82:435–441
Rosenzweig KE, Sura S, Jackson A et al (2007) Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol 35:5557–5561
Sulman EP, Komaki R, Klopp AH et al (2009) Exclusion of selective nodal irradiation is associated with minimal elective nodal failure in non-small cell lung cancer. Radiat Oncol 4:5–11
Belderbos JS, Kepka L, Kong FM et al (2008) Report from the International Atomic Energy Agency (IAEA) consultants’ meeting on elective nodal irradiation in lung cancer: non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 72:335–342
Fleckenstein J, Hellwig D, Kremp S et al (2011) F-18-FDG-PET confined radiotherapy of locally advanced NSCLC with concomitant chemotherapy: results of the PET-PLAN pilot trial. Int J Radiat Oncol Biol Phys 81:e283–289
Nestle U, Kremp S, Schaefer-Schuler A et al (2005) Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med 46:1342–1348
Chapet O, Kong FM, Quint LE et al (2005) CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys 63:170–178
Mark LB, Bentzen SM, Deasy JO et al (2010) Radiation dose-volume effects in the lung. Int J Radiation Oncol Biol Phys 76:S70–S76
Gagliardi G, Constine LS, Moiseenko V et al (2010) Radiation dose-volume effects in the heart. Int J Radiation Oncol Biol Phys 76:S77–S85
Werner-Wasik M, Yorke E, Deasy J et al (2010) Radiation dose-volume effects in the esophagus. Int J Radiation Oncol Biol Phys 76:S86–S93
ICRU. International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT) (2010) ICRU Report 83. J ICRU 10:1–106
Baltas D, Kolotas C, Geramani K et al (1998) A conformal index (COIN) to evaluate implant quality and dose specification in brachytherapy. Int J Radiat Oncol Biol Phys 40:515–524
Martel M, Ten Haken R, Hazuka M et al (1999) Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 24:31–37
Källman P, Agren A, Brahme A. (1992) Tumor and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Oncol Biol Phys 62:249–262
Bradley JD, Paulus R, Komaki R (2015) et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187–199
Shi A, Zhu G, Wu H et al (2010) Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol 5:35
Grills IS, Yan D, Martinez AA et al (2003) Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 57:875–890
Murshed H, Liu HH, Barker JL et al (2004) Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 58:1258–1267
Lievens Y, Nulens A, Gaber MA et al (2011) Intensity-modulated radiotherapy for locally advanced non-small-cell lung cancer: a dose-escalation planning study. Int J Radiat Oncol Biol Phys 80:306–313
De Ruysscher D, Faivre-Finn C, Nestle U et al (2010) European organisation for research and treatment of cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol 28:5301–5310
Yuan S, Sun X, Li M et al (2007) A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol 30:239–244
De Ruysscher D, Wanders S, van Haren E et al (2005) Selective mediastinal node irradiation based on FDG-PET scan data in patients with non – small-cell lung cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys 62:988–994
Bezjak A, Rodrigues G, Hope A et al (2012) Intensity-modulated radiotherapy in the treatment of lung cancer. Clin Oncol 24:508–520
Govaert SL, Troost EG, Schuurbiers OC et al (2012) Treatment outcome and toxicity of intensity-modulated (chemo) radiotherapy in stage III non-small cell lung cancer patients. Radiat Oncol 7:150–156
Jensen AD, Münter MW, Bischoff HG et al (2011) Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab. The NEAR trial. Cancer 117:2986–2994
De Bree I, van Hinsberg MG, van Veelen LR et al (2012) High-dose radiotherapy in inoperable nonsmall cell lung cancer: comparison of volumetric modulated arc therapy, dynamic IMRT and 3D conformal radiotherapy. Med Dosim 37:353–357
Bertelsen A, Hansen O, Brink C (2012) Does VMAT for treatment of NSCLC patients increase the risk of pneumonitis compared to IMRT?—A planning study. Acta Oncol 51:752–758
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Fleckenstein, K. Kremp, S. Kremp, J. Palm, and C. Rübe state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Rights and permissions
About this article
Cite this article
Fleckenstein, J., Kremp, K., Kremp, S. et al. IMRT and 3D conformal radiotherapy with or without elective nodal irradiation in locally advanced NSCLC. Strahlenther Onkol 192, 75–82 (2016). https://doi.org/10.1007/s00066-015-0900-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0900-9
Keywords
- Non-small cell lung carcinoma
- Intensity-modulated radiation therapy
- Conformal radiotherapy
- Positron emission tomography
- Organs at risk