Abstract
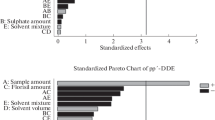
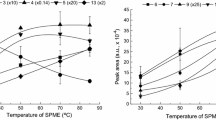
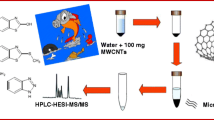
A cost-effective and low solvent consumption method, based on the matrix solid-phase dispersion (MSPD) technique, for the determination of six benzotriazole UV absorbers in sediments is presented. Sieved samples (0.5 g) were first mixed in a mortar with a solid sorbent and then transferred to a polypropylene syringe containing a layer of clean-up co-sorbent. Analytes were eluted with a suitable solvent and further determined by gas chromatography with tandem mass spectrometry (GC-MS/MS). Under final conditions, diatomaceous earth and silica, deactivated to 10%, were used as inert dispersant and clean-up co-sorbent, respectively. Analytes were recovered using just 5 mL of dichloromethane, and this extract was concentrated and exchanged to 1 mL of isooctane. Further removal of co-extracted sulphur was achieved adding activated copper powder to final extracts, which were stored overnight, before injection in the GC-MS/MS system. The accuracy of the method was assessed with river and marine sediment samples showing different carbon contents and spiked at different concentrations in the range from 40 to 500 ng g−1. Recoveries varied between 78% and 110% with associated standard deviations below 14%. The limits of quantification of the method stayed between 3 and 15 ng g−1. Levels of target compounds in sediment samples ranged from not detected up to a maximum of 56 ng g−1 for Tinuvin 328.




Similar content being viewed by others
References
Reiter SM, Buchberger W, Klampfl CW (2011) Anal Bioanal Chem 400:2317–2322
Himmelsbach M, Buchberger W, Reingruber E (2009) Polym Degrad Stabil 94:1213–1219
Gennaro MC, Gianotti V, Alberi F, Angelino S, Scagliotti M (1999) J Liq Chromatogr Relat Technol 22:2689–2700
Reddy CM, Quinn JG, King JW (2000) Environ Sci Technol 34:973–979
Hartmann PC, Quinn JG, Cairns RW, King JW (2005) Mar Pollut Bull 50:388–395
Nakata H, Murata S, Filatreau J (2009) Environ Sci Technol 43:6920–6926
Nakata H, Shinohara R, Murata S, Watanabe M (2010) J Environ Monit 12:2088–2092
Kim JW, Ramaswamy BR, Chang KH, Isobe T, Tanabe S (2011) J Chromatogr A 1218:3511–3520
Zhang Z, Ren N, Li YF, Kunisue T, Gao D, Kannan K (2011) Environ Sci Technol 45:3909–3916
Kameda Y, Kimura K, Miyazaki M (2011) Environ Pollut 159:1570–1576
Carpinteiro I, Abuín B, Rodríguez I, Cela R, Ramil M (2010) Anal Bioanal Chem 397:829–839
Carpinteiro I, Abuín B, Rodríguez I, Ramil M, Cela R (2010) J Chromatogr A 1217:3729–3735
Barker SA, Long AR, Short CR (1989) J Chromatogr A 475:353–361
Barker SA (2007) J Biochem Biophys Methods 70:151–162
García-López M, Canosa P, Rodríguez I (2008) Anal Bioanal Chem 391:963–974
Capriotti AL, Cavaliere C, Giansanti P, Gubbiotti R, Samperi R, Lagana A (2010) J Chromatogr A 1217:2521–2532
Sánchez-Brunete C, Miguel E, Tadeo JL (2007) J Chromatogr A 1148:219–227
Blanco E, Casais MC, Mejuto MC, Cela R (2006) Anal Chem 78:2772–2778
González-Mariño I, Rodríguez I, Quintana JB, Cela R (2010) Anal Bioanal Chem 398:2289–2297
Bogialli S, Curini R, Di Corcia A, Nazzari M, Samperi R (2003) Anal Chem 75:1798–1804
Parera J, Santos FJ, Galceran MT (2004) J Chromatogr A 1046:19–26
Rodil R, Moeder M (2008) Anal Chim Acta 612:152–159
Pardos M, Benninghoff C, Thomas RL, Khim-Heang S (1999) Environ Toxicol Chem 18:188–193
Acknowledgements
This study has been supported by the Xunta de Galicia, Spanish Government and E.U. FEDER funds (projects PGIDIT08MDS008CT and CTQ2009-08377). I.C. and M.R. thank their FPU and Isidro Parga Pondal contracts to the Spanish Ministry of Education and the Xunta de Galicia, respectively. We are also in debt with Prof. Ternes (BfG, Germany) for supplying some of the analysed sediment samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the 10th Anniversary Issue.
Rights and permissions
About this article
Cite this article
Carpinteiro, I., Abuín, B., Ramil, M. et al. Matrix solid-phase dispersion followed by gas chromatography tandem mass spectrometry for the determination of benzotriazole UV absorbers in sediments. Anal Bioanal Chem 402, 519–527 (2012). https://doi.org/10.1007/s00216-011-5386-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5386-4