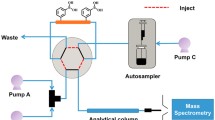
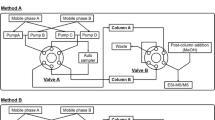
Abstract
Modified nucleosides derived from the RNA metabolism constitute an important chemical class, which are discussed as potential biomarkers in the detection of mammalian breast cancer. Not only the variability of modifications, but also the complexity of biological matrices such as urinary samples poses challenges in the analysis of modified nucleosides. In the present work, a comprehensive two-dimensional liquid chromatography mass spectrometry (2D-LC-MS) approach for the analysis of modified nucleosides in biological samples was established. For prepurification of urinary samples and cell culture supernatants, we performed a cis-diol specific affinity chromatography using boronate-derivatized polyacrylamide gel. In order to establish a 2D-LC method, we tested numerous column combinations and chromatographic conditions. In order to determine the target compounds, we coupled the 2D-LC setup to a triple quadrupole mass spectrometer performing full scans, neutral loss scans, and multiple reaction monitoring (MRM). The combination of a Zorbax Eclipse Plus C18 column with a Zorbax Bonus-RP column was found to deliver a high degree of orthogonality and adequate separation. By application of 2D-LC-MS approaches, we were able to detect 28 target compounds from RNA metabolism and crosslinked pathways in urinary samples and 26 target compounds in cell culture supernatants, respectively. This is the first demonstration of the applicability and benefit of 2D-LC-MS for the targeted metabolome analysis of modified nucleosides and compounds from crosslinked pathways in different biological matrices.






Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
McShane LM, Hayes DF (2012) Publication of tumor marker research results: the necessity for complete and transparent reporting. J Clin Oncol 30:4223–4232
Bullinger D, Frickenschmidt A, Pelzing M, Zey T, Zurek G, Laufer S, Kammerer B (2005) identification of urinary nucleosides by ESI-TOF-MS. LC-GC Europe 16–17
Bullinger D, Fux R, Nicholson G, Plontke S, Belka C, Laufer S, Gleiter CH, Kammerer B (2008) Identification of urinary modified nucleosides and ribosylated metabolites in humans via combined ESI-FTICR MS and ESI-IT MS analysis. J Am Soc Mass Spectrom 19:1500–1513
Borek E, Baliga BS, Gehrke CW, Kuo CW, Belman S, Troll W, Waalkes TP (1977) High turnover rate of transfer RNA in tumor tissue. Cancer Res 37:3362–3366
Randerath E, Chia LL, Morris HP, Randerath K (1974) Transfer RNA base composition studies in Morris hepatomas and rat liver. Cancer Res 34:643–653
Liebich HM, Muller-Hagedorn S, Klaus F, Meziane K, Kim KR, Frickenschmidt A, Kammerer B (2005) Chromatographic, capillary electrophoretic and matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis of urinary modified nucleosides as tumor markers. J Chromatogr A 1071:271–275
Colonna A, Russo T, Esposito F, Salvatore F, Cimino F (1983) Determination of pseudouridine and other nucleosides in human blood serum by high-performance liquid chromatography. Anal Biochem 130:19–26
Boschi-Muller S, Motorin Y (2013) Chemistry enters nucleic acids biology: enzymatic mechanisms of RNA modification. Biochemistry (Mosc) 78:1392–1404
Mandel LR, Srinivasan PR, Borek E (1966) Origin of urinary methylated purines. Nature 209:586–588
Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P, Dame ZT, Poelzer J, Huynh J, Yallou FS, Psychogios N, Dong E, Bogumil R, Roehring C, Wishart DS (2013) The human urine metabolome. PLoS One 8:e73076
Urakami K, Zangiacomi V, Yamaguchi K, Kusuhara M (2010) Quantitative metabolome profiling of Illicium anisatum by capillary electrophoresis time-of-flight mass spectrometry. Biomed Res 31:161–163
Frickenschmidt A, Frohlich H, Bullinger D, Zell A, Laufer S, Gleiter CH, Liebich H, Kammerer B (2008) Metabonomics in cancer diagnosis: mass spectrometry-based profiling of urinary nucleosides from breast cancer patients. Biomarkers 13:435–449
Lee SH, Jung BH, Kim SY, Chung BC (2004) A rapid and sensitive method for quantitation of nucleosides in human urine using liquid chromatography/mass spectrometry with direct urine injection. Rapid Commun Mass Spectrom 18:973–977
Dudley E, Lemiere F, Van Dongen W, Tuytten R, El-Sharkawi S, Brenton AG, Esmans EL, Newton RP (2004) Analysis of urinary nucleosides. IV. Identification of urinary purine nucleosides by liquid chromatography/electrospray mass spectrometry. Rapid Commun Mass Spectrom 18:2730–2738
Kammerer B, Frickenschmidt A, Gleiter CH, Laufer S, Liebich H (2005) MALDI-TOF MS analysis of urinary nucleosides. J Am Soc Mass Spectrom 16:940–947
Dudley E, Tuytten R, Bond A, Lemière F, Brenton AG, Esmans EL, Newton RP (2005) Study of the mass spectrometric fragmentation of pseudouridine: comparison of fragmentation data obtained by matrix-assisted laser desorption/ionisation post-source decay, electrospray ion trap multistage mass spectrometry, and by a method utilising electrospray quadrupole time-of-flight tandem mass spectrometry and in-source fragmentation. Rapid Commun Mass Spectrom 19:3075–3085
Choi BK, Hercules DM, Gusev AI (2001) LC-MS/MS signal suppression effects in the analysis of pesticides in complex environmental matrices. Fresenius J Anal Chem 369:370–377
Enke CG (1997) A predictive model for matrix and analyte effects in electrospray ionization of singly-charged ionic analytes. Anal Chem 69:4885–4893
Kebarle P, Verkerk UH (2009) Electrospray: from ions in solution to ions in the gas phase, what we know now. Mass Spectrom Rev 28:898–917
Giddings JC (2005) Concepts and comparisons in multidimensional separation. J High Resol Chromatogr 10:319–323
François I, Sandra K, Sandra P (2009) Comprehensive liquid chromatography: fundamental aspects and practical considerations—a review. Anal Chim Acta 641:14–31
Erni F, RWF (1978) Two-dimensional column liquid chromatographic technique for resolution of complex mixtures. J Chromatogr A 149:561–569
Elsner V, Laun S, Melchior D, Kohler M, Schmitz OJ (2012) Analysis of fatty alcohol derivatives with comprehensive two-dimensional liquid chromatography coupled with mass spectrometry. J Chromatogr A 1268:22–28
Kittlaus S, Schimanke J, Kempe G, Speer K (2013) Development and validation of an efficient automated method for the analysis of 300 pesticides in foods using two-dimensional liquid chromatography-tandem mass spectrometry. J Chromatogr A 1283:98–109
Montero L, Herrero M, Ibanez E, Cifuentes A (2014) Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 35:1644–1651
Stoll DR, Li X, Wang X, Carr PW, Porter SEG, Rutan SC (2007) Fast, comprehensive two-dimensional liquid chromatography. J Chromatogr A 1168:3–43
Giddings JC (1967) Maximum number of components resolvable by gel filtration and other elution chromatographic methods. Anal Chem 39:1027–1028
Li D, Schmitz OJ (2013) Use of shift gradient in the second dimension to improve the separation space in comprehensive two-dimensional liquid chromatography. Anal Bioanal Chem 405:6511–6517
Liebich HM, Di Stefano C, Wixforth A, Schmid HR (1997) Quantitation of urinary nucleosides by high-performance liquid chromatography. J Chromatogr A 763:193–197
Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FA, Fabris D, Agris PF (2011) The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res 39:D195–D201
Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, Oda Y, Kakazu Y, Kusano M, Tohge T, Matsuda F, Sawada Y, Hirai MY, Nakanishi H, Ikeda K, Akimoto N, Maoka T, Takahashi H, Ara T, Sakurai N, Suzuki H, Shibata D, Neumann S, Iida T, Funatsu K, Matsuura F, Soga T, Taguchi R, Saito K, Nishioka T (2010) MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom 45:703–714
Smith CA, O’Maille G, Want EJ, Qin C, Trauger SA, Brandon TR, Custodio DE, Abagyan R, Siuzdak G (2005) METLIN: a metabolite mass spectral database. Ther Drug Monit 27:747–751
Henneges C, Bullinger D, Fux R, Friese N, Seeger H, Neubauer H, Laufer S, Gleiter CH, Schwab M, Zell A, Kammerer B (2009) Prediction of breast cancer by profiling of urinary RNA metabolites using support vector machine-based feature selection. BMC Cancer 9:104
Hsu WY, Lin WD, Tsai Y, Lin CT, Wang HC, Jeng LB, Lee CC, Lin YC, Lai CC, Tsai FJ (2011) Analysis of urinary nucleosides as potential tumor markers in human breast cancer by high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Clin Chim Acta 412:1861–1866
Sasco AJ, Rey F, Reynaud C, Bobin JY, Clavel M, Niveleau A (1996) Breast cancer prognostic significance of some modified urinary nucleosides. Cancer Lett 108:157–162
Zheng YF, Kong HW, Xiong JH, Lv S, Xu GW (2005) Clinical significance and prognostic value of urinary nucleosides in breast cancer patients. Clin Biochem 38:24–30
Snyder LR, Dolan JW, Carr PW (2004) The hydrophobic-subtraction model of reversed-phase column selectivity. J Chromatogr A 1060:77–116
Snyder LR (1965) Principles of gradient elution. Chromatogr Rev 7:1–51
Galante RN (1986) Gradient elution in column liquid chromatography theory and practice. Journal of Chromatography Library—Vol 31. Amsterdam, The Netherlands
Jandera P (2005) Gradient elution in liquid column chromatography-prediction of retention and optimization of separation. Adv Chromatogr 43:1–108
Mayfield KJ, Shalliker RA, Catchpoole HJ, Sweeney AP, Wong V, Guiochon G (2005) Viscous fingering induced flow instability in multidimensional liquid chromatography. J Chromatogr A 1080:124–131
Dearden JC, Bentley D (2002) The components of the “critical quartet” log Kow values assessed by four commercial software packages. SAR QSAR Environ Res 13:185–197
Finizio A, Vighi M, Sandroni D (1997) Determination of n-octanol/water partition coefficient (Kow) of pesticide critical review and comparison of methods. Chemosphere 34:131–161
Jihong Liu SC (2009) A theoretical and mass spectrometry study of the novel mechanism of N-glycosidic bond cleavage in nucleoside. Int J Mass Spectrom 282:1–5
Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant HE, Bundred NJ, Burchell JM, Campbell AM, Carroll JS, Clarke RB, Coles CE, Cook GJ, Cox A, Curtin NJ, Dekker LV, Silva Idos S, Duffy SW, Easton DF, Eccles DM, Edwards DR, Edwards J, Evans D, Fenlon DF, Flanagan JM, Foster C, Gallagher WM, Garcia-Closas M, Gee JM, Gescher AJ, Goh V, Groves AM, Harvey AJ, Harvie M, Hennessy BT, Hiscox S, Holen I, Howell SJ, Howell A, Hubbard G, Hulbert-Williams N, Hunter MS, Jasani B, Jones LJ, Key TJ, Kirwan CC, Kong A, Kunkler IH, Langdon SP, Leach MO, Mann DJ, Marshall JF, Martin L, Martin SG, Macdougall JE, Miles DW, Miller WR, Morris JR, Moss SM, Mullan P, Natrajan R, O’Connor JP, O’Connor R, Palmieri C, Pharoah PD, Rakha EA, Reed E, Robinson SP, Sahai E, Saxton JM, Schmid P, Smalley MJ, Speirs V, Stein R, Stingl J, Streuli CH, Tutt AN, Velikova G, Walker RA, Watson CJ, Williams KJ, Young LS, Thompson AM (2013) Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res 15:R92
Acknowledgments
We acknowledge Ralf Falter (Agilent Technologies, Waldbronn, Germany) for the support and fruitful discussions. We would like to thank Tilman Brummer (Albert-Ludwigs-University Freiburg, Germany) for providing the breast epithelial cell line MCF-10A and the technical support.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 84 kb)
Rights and permissions
About this article
Cite this article
Willmann, L., Erbes, T., Krieger, S. et al. Metabolome analysis via comprehensive two-dimensional liquid chromatography: identification of modified nucleosides from RNA metabolism. Anal Bioanal Chem 407, 3555–3566 (2015). https://doi.org/10.1007/s00216-015-8516-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8516-6