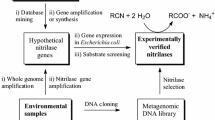
Abstract
The cyanide-degrading nitrilases are of notable interest for their potential to remediate cyanide contaminated waste streams, especially as generated in the gold mining, pharmaceutical, and electroplating industries. This review provides a brief overview of cyanide remediation in general but with a particular focus on the cyanide-degrading nitrilases. These are of special interest as the hydrolysis reaction does not require secondary substrates or cofactors, making these enzymes particularly good candidates for industrial remediation processes. The genetic approaches that have been used to date for engineering improved enzymes are described; however, recent structural insights provide a promising new approach.




Similar content being viewed by others
References
Abou Nader M (2012) Directed evolution of cyanide degrading enzymes. Doctoral dissertation Texas A&M University
Akcil A (2003) Destruction of cyanide in gold mill effluents: biological versus chemical treatments. Biotechnol Adv 21:501–511
Alphey MS, Williams RA, Mottram JC, Coombs GH, Hunter WN (2003) The crystal structure of Leishmania major 3-mercaptopyruvate sulfurtransferase: a three-domain architecture with a serine protease-like triad at the active site. J Biol Chem 278:48219–48227. doi:10.1074/jbc.M307187200
Anderson PM, Little RM (1986) Kinetic properties of cyanase. Biochemistry 25(7):1621–1626
Basile LJ, Willson RC, Sewell BT, Benedik MJ (2008) Genome mining of cyanide-degrading nitrilases from filamentous fungi. Appl Microbiol Biotechnol 80:427–435. doi:10.1007/s00253-008-1559-2
Belani KG, Singh H, Beebe DS, George P, Patterson SE, Nagasawa HT, Vince R (2012) Cyanide toxicity in juvenile pigs and its reversal by a new prodrug, sulfanegen sodium. Anesth Analg 114:956–961. doi:10.1213/ANE.0b013e31824c4eb5
Bork P, Koonin EV (1994) A new family of carbon-nitrogen hydrolases. Protein Sci 3(8):1344–1346
Botz MM (2000) Modeling of natural cyanide attenuation in tailings impoundments. Miner Mettal Proc 17:228–233
Brenner M, Kim JG, Lee J, Mahon SB, Lemor D, Ahdout R, Boss GR, Blackledge W, Jann L, Nagasawa HT, Patterson SE (2010) Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol Appl Pharmacol 248:269–276. doi:10.1016/j.taap.2010.08.002
Broderius SJ (1981) Determination of hydrocyanic acid and free cyanide in aqueous solution. Anal Chem 53:1472–1477. doi:10.1021/ac00232a040
Brysk MM, Corpe WA, Hankes LV (1969) Beta-cyanoalanine formation by Chromobacterium violaceum. J Bacteriol 97:322–327
Cipollone R, Ascenzi P, Visca P (2007) Common themes and variations in the rhodanese superfamily. IUBMB Life 59:51–59. doi:10.1080/15216540701206859
Cipollone R, Ascenzi P, Tomao P, Imperi F, Visca P (2008) Enzymatic detoxification of cyanide: clues from Pseudomonas aeruginosa rhodanese. J Mol Microbiol Biotechnol 15:199–211. doi:10.1159/000121331
Crum MA, Park JM, Mulelu AE, Sewell BT, Benedik MJ (2015a) Probing C-terminal interactions of the Pseudomonas stutzeri cyanide-degrading CynD protein. Appl Microbiol Biotechnol 99:3093–3102. doi:10.1007/s00253-014-6335-x
Crum MA, Park JM, Sewell BT, Benedik MJ (2015b) C-terminal hybrid mutant of Bacillus pumilus cyanide dihydratase dramatically enhances thermal stability and pH tolerance by reinforcing oligomerization. J Appl Microbiol 118:881–889. doi:10.1111/jam.12754
Crum MA, Sewell BT, Benedik MJ (2016) Bacillus pumilus cyanide dihydratase mutants with higher catalytic activity. Front Microbiol 7:1264. doi:10.3389/fmicb.2016.01264
Cummings TF (2004) The treatment of cyanide poisoning. Occup Med (Lond) 54(2):82–85
Dash RR, Gaur A, Balomajumder C (2009) Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater 163:1–11. doi:10.1016/j.jhazmat.2008.06.051
Dent KC, Weber BW, Benedik MJ, Sewell BT (2009) The cyanide hydratase from Neurospora crassa forms a helix which has a dimeric repeat. Appl Microbiol Biotechnol 82:271–278. doi:10.1007/s00253-008-1735-4
Devuyst EA, Conard BR, Vergunst R, Tandi B (1989) A cyanide removal process using sulfur dioxide and air. JOM 41:43–45. doi:10.1007/BF03220847
Dorr PK, Knowles CJ (1989) Cyanide oxygenase and cyanase activities of Pseudomonas fluorescens NCIMB 11764. FEMS Microbiol Lett 60:289–294
Dunnill PM, Fowden L (1965) Enzymatic formation of beta-cyanoalanine from cyanide by Escherichia coli extracts. Nature 208:1206–1207
Fernandes BCM, Mateo C, Kiziak C, Chmura A, Wacker J, van Rantwijk F, Stolz A, Sheldon RA (2006) Nitrile hydratase activity of a recombinant nitrilase. Adv Synth Catal 348:2597–2603. doi:10.1002/adsc.200600269
Fernandez RF, Kunz DA (2005) Bacterial cyanide oxygenase is a suite of enzymes catalyzing the scavenging and adventitious utilization of cyanide as a nitrogenous growth substrate. J Bacteriol 187:6396–6402. doi:10.1128/jb.187.18.6396-6402.2005
Fernandez RF, Dolghih E, Kunz DA (2004) Enzymatic assimilation of cyanide via pterin-dependent oxygenolytic cleavage to ammonia and formate in Pseudomonas fluorescens NCIMB 11764. Appl Environ Microbiol 70:121–128
Fry WE, Munch DC (1975) Hydrogen-cyanide detoxification by Gloeocercospora sorghi. Physiol Plant Pathol 7:23–33. doi:10.1016/0048-4059(75)90056-9
Gantzer CJ (1990) Biological degradation of cyanide by nitrogen-fixing cyanobacteria. U.S. Environmental Protection Agency, Risk Reduction Engineering Laboratory, Cincinnati, OH
Gong JS, Lu ZM, Li H, Shi JS, Zhou ZM, Xu ZH (2012) Nitrilases in nitrile biocatalysis: recent progress and forthcoming research. Microb Cell Factories 11. doi:10.1186/1475-2859-11-142
Gordon RD, Qiu W, Romanov V, Lam K, Soloveychik M, Benetteraj D, Battaile KP, Chirgadze YN, Pai EF, Chirgadze NY (2013) Crystal structure of the CN-hydrolase SA0302 from the pathogenic bacterium Staphylococcus aureus belonging to the Nit and NitFhit branch of the nitrilase superfamily. J Biomol Struct Dyn 31:1057–1065. doi:10.1080/07391102.2012.719111
Gracia R, Shepherd G (2004) Cyanide poisoning and its treatment. Pharmacotherapy 24:1358–1365
Gupta N, Balomajumder C, Agarwal VK (2010) Enzymatic mechanism and biochemistry for cyanide degradation: a review. J Hazard Mater 176:1–13. doi:10.1016/j.jhazmat.2009.11.038
Hardy RW, Knight E Jr (1967) ATP-dependent reduction of azide and HCN by N2-fixing enzymes of Azotobacter vinelandii and Clostridium pasteurianum. Biochim Biophys Acta 139:69–90
Harris RE, Knowles CJ (1983a) Isolation and growth of a Pseudomonas species that utilizes cyanide as a source of nitrogen. J Gen Microbiol 129:1005–1011
Harris RE, Knowles CJ (1983b) The conversion of cyanide to ammonia by extracts of a strain of Pseudomonas fluorescens that utilizes cyanide as a source of nitrogen for growth. FEMS Microbiol Lett 20:337–341
Hatzfeld Y, Maruyama A, Schmidt A, Noji M, Ishizawa K, Saito K (2000) Beta-cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and Arabidopsis. Plant Physiol 123:1163–1171
Hook RH, Robinson WG (1964) Ricinine nitrilase. II. Purification and properties. J Biol Chem 239:4263–4267
Howden AJM, Jill Harrison C, Preston GM (2009) A conserved mechanism for nitrile metabolism in bacteria and plants. Plant J 57(2):243–253. doi:10.1111/j.1365-313X.2008.03682.x
Igvorsen K, Hojer-Pedersen B, Godtfredsen SE (1991) Novel cyanide-hydrolyzing enzyme from Alcaligenes xylosoxidans subsp. denitrificans. Appl Environ Microbiol 57:1783–1789
Jandhyala D, Berman M, Meyers PR, Sewell BT, Willson RC, Benedik MJ (2003) CynD, the cyanide dihydratase from Bacillus pumilus: gene cloning and structural studies. Appl Environ Microbiol 69:4794–4805
Jandhyala DM, Willson RC, Sewell BT, Benedik MJ (2005) Comparison of cyanide-degrading nitrilases. Appl Microbiol Biotechnol 68:327–335. doi:10.1007/s00253-005-1903-8
Jenrich R, Trompetter I, Bak S, Olsen CE, Moller BL, Piotrowski M (2007) Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proc Natl Acad Sci U S A 104:18848–18853. doi:10.1073/Pnas.0709315104
Kao CM, Liu JK, Lou HR, Lin CS, Chen SC (2003) Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 50:1055–1061
Keusgen M, Kloock JP, Knobbe DT, Jünger M, Krest I, Goldbach M, Klein W, Schöning MJ (2004) Direct determination of cyanides by potentiometric biosensors. Sensors Actuators B Chem 103:380–385. doi:10.1016/j.snb.2004.04.067
Kimani SW, Agarkar VB, Cowan DA, Sayed MF, Sewell BT (2007) Structure of an aliphatic amidase from Geobacillus pallidus RAPc8. Acta Crystallogr D Biol Crystallogr 63(Pt 10):1048–1058. doi:10.1107/S090744490703836X
Kiziak C, Stolz A (2009) Identification of amino acid residues responsible for the enantioselectivity and amide formation capacity of the arylacetonitrilase from Pseudomonas fluorescens EBC191. Appl Environ Microbiol 75:5592–5599. doi:10.1128/aem.00301-09
Knorre H, Griffiths A, Degussa A (1984) Cyanide detoxification with hydrogen peroxide using the Degussa process. Paper presented at the Conference on Cyanide and the Environment, Tucson Arizona, U S A
Kobayashi M, Goda M, Shimizu S (1999) Nitrilase catalyzes amide hydrolysis as well as nitrile hydrolysis. Biochem Biophys Res Commun 253:662–666
Kobayashi M, Komeda H, Yanaka N, Nagasawa T, Yamada H (1992) Nitrilase from Rhodococcus rhodochrous J1—sequencing and overexpression of the gene and identification of an essential cysteine residue. J Biol Chem 267:20746–20751
Kovac C (2000) Cyanide spill threatens health in Hungary. BMJ 320:536
Kuhn DD, Young TC (2005) Photolytic degradation of hexacyanoferrate (II) in aqueous media: the determination of the degradation kinetics. Chemosphere 60:1222–1230. doi:10.1016/j.chemosphere.2005.02.011
Kumaran D, Eswaramoorthy S, Gerchman SE, Kycia H, Studier FW, Swaminathan S (2003) Crystal structure of a putative CN hydrolase from yeast. Proteins Struct Funct Genet 52:283–291. doi:10.1002/Prot.10417
Kunz DA, Fernandez RF, Parab P (2001) Evidence that bacterial cyanide oxygenase is a pterin-dependent hydroxylase. Biochem Biophys Res Commun 287:514–518. doi:10.1006/bbrc.2001.5611
Kunz DA, Nagappan O (1989) Cyanase-mediated utilization of cyanate in Pseudomonas fluorescens NCIB 11764. Appl Environ Microbiol 55:256–258
Kunz DA, Wang CS, Chen JL (1994) Alternative routes of enzymic cyanide metabolism in Pseudomonas fluorescens NCIMB 11764. Microbiology 140:1705–1712. doi:10.1099/13500872-140-7-1705
Li J, Burgess BK, Corbin JL (1982) Nitrogenase reactivity: cyanide as substrate and inhibitor. Biochemistry 21:4393–4402. doi:10.1021/bi00261a031
Lundgren S, Lohkamp B, Andersen B, Piskur J, Dobritzsch D (2008) The crystal structure of beta-alanine synthase from Drosophila melanogaster reveals a homooctameric helical turn-like assembly. J Mol Biol 377:1544–1559. doi:10.1016/j.jmb.2008.02.011
Macadam AM, Knowles CJ (1984) Purification and properties of β-cyano-l-alanine synthase from the cyanide-producing bacterium, Chromobacterium violaceum. Biochim Biophys Acta Protein Struct Mol Enzymol 786:123–132. doi:10.1016/0167-4838(84)90081-5
Mahadevan S, Thimann KV (1964) Nitrilase. II. Substrate specificity and possible mode of action. Arch Biochem Biophys 107:62–68
Martínková L, Veselá AB, Rinágelová A, Chmátal M (2015) Cyanide hydratases and cyanide dihydratases: emerging tools in the biodegradation and biodetection of cyanide. Appl Microbiol Biotechnol 99:8875–8882
Materassi R, Balloni W, Florenzano G (1977) Cyanide reduction by nitrogenase in intact cells of Rhodopseudomonas gelatinose Molisch. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene Zweite naturwissenschaftliche Abt: Allgemeine, landwirtschaftliche und technische Mikrobiologie 132:413–417
Meyers PR, Gokool P, Rawlings DE, Woods DR (1991) An efficient cyanide-degrading Bacillus pumilus strain. J Gen Microbiol 137:1397–1400
Meyers PR, Rawlings DE, Woods DR, Lindsey GG (1993) Isolation and characterization of a cyanide dihydratase from Bacillus pumilus C1. J Bacteriol 175:6105–6112
Michaels R, Corpe WA (1965) Cyanide formation by Chromobacterium violaceum. J Bacteriol 89:106–112
Miller JM, Conn EE (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65:1199–1202
Mosher JB, Figueroa L (1996) Biological oxidation of cyanide: a viable treatment option for the minerals processing industry? Miner Eng 9:573–581. doi:10.1016/0892-6875(96)00044-1
Mueller P, Egorova K, Vorgias CE, Boutou E, Trauthwein H, Verseck S, Antranikian G (2006) Cloning, overexpression, and characterization of a thermoactive nitrilase from the hyperthermophilic archaeon Pyrococcus abyssi. Protein Expr Purif 47:672–681. doi:10.1016/J.Pep.2006.01.006
Mundy BP, Liu FH, Strobel GA (1973) Alpha-aminobutyronitrile as an intermediate in cyanide fixation by Rhizoctonia solani. Can J Biochem 51:1440–1442
Nagasawa T, Wieser M, Nakamura T, Iwahara H, Yoshida T, Gekko K (2000) Nitrilase of Rhodococcus rhodochrous J1. Conversion into the active form by subunit association. Eur J Biochem 267:138–144
Nakai T, Hasegawa T, Yamashita E, Yamamoto M, Kumasaka T, Ueki T, Nanba H, Ikenaka Y, Takahashi S, Sato M, Tsukihara T (2000) Crystal structure of N-carbamyl-D-amino acid amidohydrolase with a novel catalytic framework common to amidohydrolases. Struct Fold Des 8:729–737. doi:10.1016/S0969-2126(00)00160-X
Ogunlabi OO, Agboola FK (2007) A soluble beta-cyanoalanine synthase from the gut of the variegated grasshopper Zonocerus variegatus (L.) Insect Biochem Mol Biol 37:72–79. doi:10.1016/j.ibmb.2006.10.003
O'Reilly C, Turner PD (2003) The nitrilase family of CN hydrolysing enzymes - a comparative study. J Appl Microbiology 95(6):1161-1174
Pace HC, Brenner C (2001) The nitrilase superfamily: classification, structure and function. Genome Biol 2:reviews0001.1–reviews0001.9
Pace HC, Hodawadekar SC, Draganescu A, Huang J, Bieganowski P, Pekarsky Y, Croce CM, Brenner C (2000) Crystal structure of the worm NitFhit Rosetta stone protein reveals a Nit tetramer binding two Fhit dimers. Curr Biol 10:907–917. doi:10.1016/S0960-9822(00)00621-7
Padmaja G, Balagopal C (1985) Cyanide degradation by Rhizopus oryzae. Can J Microbiol 31:663–669. doi:10.1139/m85-126
Park JM, Mulelu A, Sewell BT, Benedik MJ (2016) Probing an interfacial surface in the cyanide dihydratase from Bacillus pumilus, a spiral forming nitrilase. Front Microbiol 6:1479. doi:10.3389/fmicb.2015.01479
Piotrowski M, Schonfelder S, Weiler EW (2001) The Arabidopsis thaliana isogene NIT4 and its orthologs in tobacco encode beta-cyano-L-alanine hydratase/nitrilase. J Biol Chem 276:2616–2621. doi:10.1074/jbc.M007890200
Poole CJ, Kind PR (1986) Deficiency of thiosulphate sulphurtransferase (rhodanese) in Leber’s hereditary optic neuropathy. Br Med J 292:1229–1230
Porter N, Knowles CJ (1979) Cyanide-resistant growth in Citrobacter Freundii and other Enterobacteriaceae. FEMS Microbiol Lett 5:323–326. doi:10.1111/j.1574-6968.1979.tb03331.x
Qian D, Jiang L, Lu L, Wei C, Li Y (2011) Biochemical and structural properties of cyanases from Arabidopsis thaliana and Oryza sativa. PLoS One 6:e18300. doi:10.1371/journal.pone.0018300
Rico M, Benito G, Salgueiro AR, Diez-Herrero A, Pereira HG (2008) Reported tailings dam failures: a review of the European incidents in the worldwide context. J Hazard Mater 152:846–852. doi:10.1016/j.jhazmat.2007.07.050
Ritcey GM (2005) Tailings management in gold plants. Hydrometallurgy 78:3–20. doi:10.1016/j.hydromet.2005.01.001
Robey H (2004) Thermal hydrolysis of cyanide. Pollut Eng 36:22
Rodgers PB, Knowles CJ (1978) Cyanide production and degradation during growth of Chromobacterium violaceum. Microbiology 108:261–267. doi:10.1099/00221287-108-2-261
Sewell BT, Berman MN, Meyers PR, Jandhyala D, Benedik MJ (2003) The cyanide degrading nitrilase from Pseudomonas stutzeri AK61 is a two-fold symmetric, 14-subunit spiral. Structure 11:1413–1422
Sewell BT, Thuku RN, Zhang X, Benedik MJ (2005) Oligomeric structure of nitrilases: effect of mutating interfacial residues on activity. Ann N Y Acad Sci 1056:153–159
Soriano-Maldonado P, Isabel Martinez-Gomez A, Andujar-Sanchez M, Neira JL, Maria Clemente-Jimenez J, Las Heras-Vazquez FJ, Rodriguez-Vico F, Martinez-Rodriguez S (2011) Biochemical and mutational studies of the Bacillus cereus CECT 5050T formamidase support the existence of a C-E-E-K tetrad in several members of the nitrilase superfamily. Appl Environ Microbiol 77:5761–5769. doi:10.1128/aem.00312-11
Stevenson DE, Feng R, Dumas F, Groleau D, Mihoc A, Storer AC (1992) Mechanistic and structural studies on Rhodococcus ATCC-39484 nitrilase. Biotechnol Appl Biochem 15:283–302
Stuehr J, Yeager E, Sachs T, Hovorka F (1963) Ultrasonic investigation of the rate of hydrolysis of potassium cyanide. J Chem Phys 38:587–593. doi:10.1063/1.1733708
Thimann KV, Mahadevan S (1964) Nitrilase. I. Occurrence, preparation and general properties of the enzyme. Arch Biochem Biophys 105:133–141
Thuku RN, Brady D, Benedik MJ, Sewell BT (2009) Microbial nitrilases: versatile, spiral forming, industrial enzymes. J Appl Microbiol 106:703–727. doi:10.1111/j.1365-2672.2008.03941.x
Thuku RN, Weber BW, Varsani A, Sewell BT (2007) Post-translational cleavage of recombinantly expressed nitrilase from Rhodococcus rhodochrous J1 yields a stable, active helical form. FEBS J 274:2099–2108. doi:10.1111/j.1742-4658.2007.05752.x
Vejvoda V, Kaplan O, Bezouska K, Pompach P, Sulc M, Cantarella M, Benada O, Uhnakova B, Rinagelova A, Lutz-Wahl S, Fischer L, Kren V, Martinkova L (2008) Purification and characterization of a nitrilase from Fusarium solani O1. J Mol Catal B Enzym 50:99–106. doi:10.1016/J.Molcatb.2007.09.006
Vogel SN, Sultan TR, Ten Eyck RP (1981) Cyanide poisoning. Clin Toxicol 18:367–383. doi:10.3109/15563658108990043
Wang C, Kunz DA, Venables BJ (1996) Incorporation of molecular oxygen and water during enzymatic oxidation of cyanide by Pseudomonas fluorescens NCIMB 11764. Appl Environ Microbiol 62:2195–2197
Wang L, Watermeyer JM, Mulelu AE, Sewell BT, Benedik MJ (2012) Engineering pH-tolerant mutants of a cyanide dihydratase. Appl Microbiol Biotechnol 94:131–140. doi:10.1007/s00253-011-3620-9
Wang WC, Hsu WH, Chien FT, Chen CY (2001) Crystal structure and site-directed mutagenesis studies of N-carbamoyl-D-amino-acid amidohydrolase from Agrobacterium radiobacter reveals a homotetramer and insight into a catalytic cleft. J Mol Biol 306:251–261. doi:10.1006/jmbi.2000.4380
Watanabe A, Yano K, Ikebukuro K, Karube I (1998) Cyanide hydrolysis in a cyanide-degrading bacterium, Pseudomonas stutzeri AK61, by cyanidase. Microbiology 144:1677–1682
Weber BW, Kimani SW, Varsani A, Cowan DA, Hunter R, Venter GA, Gumbart JC, Sewell BT (2013) The mechanism of the amidases: mutating the glutamate adjacent to the catalytic triad inactivates the enzyme due to substrate mispositioning. J Biol Chem 288:28514–28523. doi:10.1074/jbc.M113.503284
Williamson DS, Dent KC, Weber BW, Varsani A, Frederick J, Thuku RN, Cameron RA, van Heerden JH, Cowan DA, Sewell BT (2010) Structural and biochemical characterization of a nitrilase from the thermophilic bacterium, Geobacillus pallidus RAPc8. Appl Microbiol Biotechnol 88:143–153. doi:10.1007/S00253-010-2734-9
Woodward JD (2011) The relationship between structure and specificity in the plant nitrilases. Doctoral dissertation, University of Cape Town
Woodward JD, Weber BW, Scheffer MP, Benedik MJ, Hoenger A, Sewell BT (2008) Helical structure of unidirectionally shadowed metal replicas of cyanide hydratase from Gloeocercospora sorghi. J Struct Biol 161:111–119. doi:10.1016/J.Jsb.2007.09.019
Zhang LJ, Yin B, Wang C, Jiang SQ, Wang HL, Yuan YA, Wei DZ (2014) Structural insights into enzymatic activity and substrate specificity determination by a single amino acid in nitrilase from Synechocystis sp PCC6803. J Struct Biol 188:93–101. doi:10.1016/j.jsb.2014.10.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Welch Foundation (A1310), the Texas Hazardous Waste Research Center (513TAM0032H), and the National Research Foundation of South Africa.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Park, J.M., Trevor Sewell, B. & Benedik, M.J. Cyanide bioremediation: the potential of engineered nitrilases. Appl Microbiol Biotechnol 101, 3029–3042 (2017). https://doi.org/10.1007/s00253-017-8204-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8204-x