Abstract
Purpose
To address tolerability and a possible pharmacologic interaction of capecitabine with sorafenib.
Methods
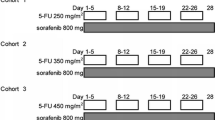
Patients with advanced solid tumors (ECOG PS 0-1) were included. Cohort A received capecitabine 750 mg/m2 BID and Cohort B received capecitabine 1,000 mg/m2 BID, both for 14 days of a 21-day cycle. Steady-state PK was obtained for capecitabine alone, sorafenib alone, and in combination. Cohort C explored an alternate schedule of 7-day on/7-day off flat dose capecitabine 1,000 mg BID with continuous dosing of sorafenib 400 mg BID.
Results
A total of 32 patients were enrolled between February 08 and April 09. Hand-foot skin reaction (HFSR) was the primary toxicity with 16 (50%) of the 32 patients experiencing grade 3 events (75% occurring during cycles 1–2). Grade 3 HFSR defined the maximum tolerated dose (MTD) of Cohort C at 1,000 mg BID flat dose of capecitabine. Other grade 3/4 toxicities were rare (diarrhea 6%, mucositis 3%, and fatigue 3%). Capecitabine did not change the C max or AUC(0-12) of sorafenib. Co-administration of sorafenib with capecitabine 750 mg/m2 (n = 6 patients) increased capecitabine AUC(0–12) 15% and produced no change in the 5FU AUC(0–12). At the capecitabine 1,000 mg/m2 dose level (n = 12 pts), there was a 16% increase in capecitabine AUC(0–12) and an 8% increase in 5FU AUC(0–12). However, these trends were not statistically significant.
Conclusions
Co-administration of sorafenib resulted in a mild increase in capecitabine AUC, although not statistically significant. Capecitabine did not affect the exposure of sorafenib. The rate of grade 3 HFSR is concerning and limits the feasibility of prolonged dosing of sorafenib with capecitabine 1,000 mg/m2 on the 21-day schedule.
Similar content being viewed by others
References
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Jemal A, Murray T, Ward E et al (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109
Xeloda® Tablets package insert. New Jersey Roche Laboratories, Inc.:April 2006
Bajetta E, Procopio G, Celio L et al (2005) Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol 23:2155–2161
Theodoulou M, Dugan U, Feigin K et al (2008) A novel capecitabine dosing schedule combined with bevacizumab is safe and active in patients with metastatic breast cancer: a phase II study. J Clin Oncol 26:66s Abstract #1101
Baselga J, Segalla JGM, Roche H et al (2009) SOLTI-0701: a double-blind, randomized phase 2b study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with capecitabine (CAP) in patients with locally advanced breast cancer. Eur J Cancer (ECCO-EMSO) 7:3–4
Awada A, Gil T, Whenham N et al (2009) Phase I dose escalation trial evaluating the safety and pharmacokinetics of sorafenib combined with capecitabine in patients with advanced solid tumors. Eur J Cancer (EORTC-NCI-AACR) 4:33 (Abstract #98)
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National cancer institute of the United States, National cancer institute of Canada. J Natl Cancer Inst 92:205–216
Lacouture ME, Wu S, Robert C et al (2008) Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 13:1001–1011
Gressett SM, Stanford BL, Hardwicke F (2006) Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract 12:131–141
Haller DG, Cassidy J, Clarke SJ et al (2008) Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol 26:2118–2123
Chiorean EG, Sweeney CJ, Verschraegen CF et al (2007) Tolerability/safety of sunitinib (SU) on schedule 2/1 in combination with capecitabine (C) in patients (pts) with advanced solid tumors (STs): a phase I dose-finding study. ASCO Meeting Abstracts 26:3562
Nexavar® Tablets Package Insert. New Jersey Bayer HealthCare Pharmaceuticals, Inc.:2009
Sweeney C, Verschraegen C, Chiorean G et al (2007) A phase I dose escalation study of sunitinib plus capecitabine in patients with advanced solid tumors. J Clin Oncol 25(18):3592
Miller KD, Chap LI, Holmes FA et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Saltz LB, Clarke S, Diaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Hochster HS, Hart LL, Ramanathan RK et al (2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 26:3523–3529
Bhargava P, Esteves B, Nosov DA et al (2009) Updated activity and safety results of a phase II randomized discontinuation trial (RDT) of AV-951, a potent and selective VEGFR1, 2, and 3 kinase inhibitor, in patients with renal cell carcinoma (RCC). J Clin Oncol (ASCO Meeting Abstracts) 27:5032
Sharma S, Abhyankar V, Burgess RE et al (2009) A phase I study of axitinib (AG-013736) in combination with bevacizumab plus chemotherapy or chemotherapy alone in patients with metastatic colorectal cancer and other solid tumors. Ann Oncol
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by a grant from Bayer Healthcare and Onyx Pharmaceuticals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Infante, J.R., Jones, S.F., Bendell, J.C. et al. A drug interaction study evaluating the pharmacokinetics and toxicity of sorafenib in combination with capecitabine. Cancer Chemother Pharmacol 69, 137–144 (2012). https://doi.org/10.1007/s00280-011-1674-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1674-0