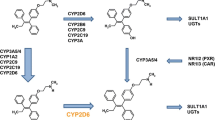
Abstract
Background
Despite decades of clinical success, tamoxifen therapy is complicated by inter-individual variability due to CYP450 polymorphism and resistance attributed to ERα/HER2 crosstalk. Direct administration of endoxifen shows promise in circumventing obligatory CYP450 bioactivation while maintaining efficacy. Separately, disruption of the crosstalk using probe antagonists against ERα (tamoxifen) and HER2 (e.g., lapatinib) has been explored clinically. However, the efficacy of this combination may be confounded by lapatinib, a potent inactivator of CYP3A4/5 which could negate the bioactivation of tamoxifen to the active metabolite endoxifen. Additionally, in a manner analogous to tamoxifen, endoxifen is similarly not immune to the development of ERα/HER2 crosstalk that could result in resistance. Simultaneous antagonism of ERα and HER2 using endoxifen and lapatinib could overcome these problems.
Methods
Metabolism studies were performed in human liver microsomes to determine the extent of inhibition of tamoxifen bioactivation by lapatinib. Synergism of endoxifen and lapatinib was assessed using the combination index design in a panel of cell models exhibiting either a priori ERα/HER2 crosstalk (BT474) or acquired ERα/HER2 crosstalk (TAM-R and MCF-7/HER2).
Results
Lapatinib inhibited tamoxifen bioactivation by up to 1.8-fold. Synergistic activity was uncovered for lapatinib and endoxifen against BT474, TAM-R and MCF-7/HER2 models of ERα/HER2 crosstalk. Western blot confirmed that endoxifen and lapatinib disrupted this crosstalk.
Conclusion
This forward-looking study extends the success of tamoxifen by exploring the effectiveness of combining the next-generation tamoxifen derivative, endoxifen with an anti-HER2 agent to combat ERα/HER2 crosstalk, and at the same time provides a solution to the predicted pharmacokinetic antagonism between lapatinib and tamoxifen.










Similar content being viewed by others
Abbreviations
- ERα:
-
Estrogen receptor α
- HER2:
-
Human epidermal growth factor receptor 2
- CYP3A4/5:
-
Cytochrome P450 3A4/3A5
- 4-OHT:
-
4-Hydroxytamoxifen
- NDMT:
-
N-desmethyltamoxifen
- CYP2D6:
-
Cytochrome P450 2D6
- CYP450:
-
Cytochrome P450
- HLM:
-
Human liver microsomes
- CLint :
-
Intrinsic clearance
- IS:
-
Internal standard
- LC/MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- DRI50 :
-
Dose reduction index at 50% cytotoxicity
- ANOVA:
-
One-way analysis of variance
- f a :
-
Fraction affected
- PI3K/Akt:
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B
References
Jordan VC (2006) Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol 147(Suppl 1):S269–S276. doi:10.1038/sj.bjp.0706399
Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80(1):61–74. doi:10.1016/j.clpt.2006.03.013
Lu WJ, Xu C, Pei Z, Mayhoub AS, Cushman M, Flockhart DA (2012) The tamoxifen metabolite norendoxifen is a potent and selective inhibitor of aromatase (CYP19) and a potential lead compound for novel therapeutic agents. Breast Cancer Res Treat 133(1):99–109. doi:10.1007/s10549-011-1699-4
Allen KE, Clark ER, Jordan VC (1980) Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure-activity relationships. Br J Pharmacol 71(1):83–91
Reid JM, Goetz MP, Kumar S, McGovern RM, Buhrow SA, Safgren SL, Suman VJ, Docktor TJ, Erlichman C, Streicher H, Doroshow JH, Collins J, Ames MM (2014) Abstract 4631: pharmacokinetics and in vivo metabolism of Z-endoxifen: results from two phase I studies in women with ER+ breast cancer, gynecologic malignancies and desmoids. Cancer Res 74(19 Supplement):4631. doi:10.1158/1538-7445.am2014-4631
Lv W, Liu J, Lu D, Flockhart DA, Cushman M (2013) Synthesis of mixed (E, Z)-, (E)-, and (Z)-norendoxifen with dual aromatase inhibitory and estrogen receptor modulatory activities. J Med Chem 56(11):4611–4618. doi:10.1021/jm400364h
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85(2):151–159. doi:10.1023/B:BREA.0000025406.31193.e8
Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenom J 5(1):6–13. doi:10.1038/sj.tpj.6500285
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E (2001) Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27(4):383–391. doi:10.1038/86882
Elens L, van Gelder T, Hesselink DA, Haufroid V, van Schaik RH (2013) CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics 14(1):47–62. doi:10.2217/pgs.12.187
Fleeman N, Martin Saborido C, Payne K, Boland A, Dickson R, Dundar Y, Fernández-Santander A, Howell S, Newman W, Oyee J (2011) The clinical effectiveness and cost-effectiveness of genotyping for CYP2D6 for the management of women with breast cancer treated with tamoxifen: a systematic review. Health Technol Assess 15(33):1–102
Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res BCR 13(4):215. doi:10.1186/bcr2889
Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A (2012) Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev 38(6):698–707. doi:10.1016/j.ctrv.2011.11.005
Early Breast Cancer Trialists’ Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
Ring A, Dowsett M (2004) Mechanisms of tamoxifen resistance. Endocr Relat Cancer 11(4):643–658
Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, Dowsett M, Johnston SR (2010) Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res Off J Am Assoc Cancer Res 16(5):1486–1497. doi:10.1158/1078-0432.CCR-09-1764
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96(12):926–935
Chu I, Blackwell K, Chen S, Slingerland J (2005) The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res 65(1):18–25
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A, Revil C, Jones A (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol Off J Am Soc Clin Oncol 27(33):5529–5537. doi:10.1200/JCO.2008.20.6847
Giuliano M, Trivedi MV, Schiff R (2013) Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care 8(4):256–262. doi:10.1159/000354253
Viedma-Rodriguez R, Baiza-Gutman L, Salamanca-Gomez F, Diaz-Zaragoza M, Martinez-Hernandez G, Ruiz Esparza-Garrido R, Velazquez-Flores MA, Arenas-Aranda D (2014) Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review). Oncol Rep 32(1):3–15. doi:10.3892/or.2014.3190
Chan EC, New LS, Chua TB, Yap CW, Ho HK, Nelson SD (2012) Interaction of lapatinib with cytochrome P450 3A5. Drug Metab Dispos Biol Fate Chem 40(7):1414–1422. doi:10.1124/dmd.112.044958
Takakusa H, Wahlin MD, Zhao C, Hanson KL, New LS, Chan EC, Nelson SD (2011) Metabolic intermediate complex formation of human cytochrome P450 3A4 by lapatinib. Drug Metab Dispos Biol Fate Chem 39(6):1022–1030. doi:10.1124/dmd.110.037531
Goetz M, Suman V, Reid J, Northfelt D, Mahr M, Dockter T, Haluska P, Kuffel M, Burhow S, Safgren S, McGovern R, Collins J, Streicher H, Hawse J, Erlichman C, Ingle J, Ames M (2013) Abstract PD3-4: a first-in-human phase I study of the tamoxifen (TAM) metabolite, Z-endoxifen hydrochloride (Z-Endx) in women with aromatase inhibitor (AI) refractory metastatic breast cancer (MBC) (NCT01327781). Cancer Res 73(24 Supplement):PD3–PD4. doi:10.1158/0008-5472.sabcs13-pd3-4
Chou TC, Talalay P (1984) Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58(3):621–681. doi:10.1124/pr.58.3.10
Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI (2003) Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144(3):1032–1044. doi:10.1210/en.2002-220620
Warburton C, Dragowska WH, Gelmon K, Chia S, Yan H, Masin D, Denyssevych T, Wallis AE, Bally MB (2004) Treatment of HER-2/neu overexpressing breast cancer xenograft models with trastuzumab (Herceptin) and gefitinib (ZD1839): drug combination effects on tumor growth, HER-2/neu and epidermal growth factor receptor expression, and viable hypoxic cell fraction. Clin Cancer Res Off J Am Assoc Cancer Res 10(7):2512–2524
Fumoleau P, Koch KM, Brain E, Lokiec F, Rezai K, Awada A, Hayward L, Werutsky G, Bogaerts J, Marreaud S, Cardoso F (2014) A phase I pharmacokinetics study of lapatinib and tamoxifen in metastatic breast cancer (EORTC 10053 Lapatam study). Breast 23(5):663–669. doi:10.1016/j.breast.2014.07.003
Hudachek SF, Gustafson DL (2013) Physiologically based pharmacokinetic model of lapatinib developed in mice and scaled to humans. J Pharmacokinet Pharmacodyn 40(2):157–176. doi:10.1007/s10928-012-9295-8
Klein DJ, Thorn CF, Desta Z, Flockhart DA, Altman RB, Klein TE (2013) PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genom 23(11):643–647. doi:10.1097/FPC.0b013e3283656bc1
Aueviriyavit S, Kobayashi K, Chiba K (2010) Species differences in mechanism-based inactivation of CYP3A in humans, rats and mice. Drug Metab Pharmacokinet 25(1):93–100
Rusnak DW, Affleck K, Cockerill SG, Stubberfield C, Harris R, Page M, Smith KJ, Guntrip SB, Carter MC, Shaw RJ, Jowett A, Stables J, Topley P, Wood ER, Brignola PS, Kadwell SH, Reep BR, Mullin RJ, Alligood KJ, Keith BR, Crosby RM, Murray DM, Knight WB, Gilmer TM, Lackey K (2001) The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res 61(19):7196–7203
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL (2002) Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21(41):6255–6263. doi:10.1038/sj.onc.1205794
Coezy E, Borgna JL, Rochefort H (1982) Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res 42(1):317–323
Katzenellenbogen BS, Norman MJ, Eckert RL, Peltz SW, Mangel WF (1984) Bioactivities, estrogen receptor interactions, and plasminogen activator-inducing activities of tamoxifen and hydroxy-tamoxifen isomers in MCF-7 human breast cancer cells. Cancer Res 44(1):112–119
Gonzalez-Malerva L, Park J, Zou L, Hu Y, Moradpour Z, Pearlberg J, Sawyer J, Stevens H, Harlow E, LaBaer J (2011) High-throughput ectopic expression screen for tamoxifen resistance identifies an atypical kinase that blocks autophagy. Proc Natl Acad Sci USA 108(5):2058–2063. doi:10.1073/pnas.1018157108
Weng SC, Kashida Y, Kulp SK, Wang D, Brueggemeier RW, Shapiro CL, Chen CS (2008) Sensitizing estrogen receptor-negative breast cancer cells to tamoxifen with OSU-03012, a novel celecoxib-derived phosphoinositide-dependent protein kinase-1/Akt signaling inhibitor. Mol Cancer Ther 7(4):800–808. doi:10.1158/1535-7163.MCT-07-0434
Schweikart KM, Eldridge SR, Safgren SL, Parman T, Reid JM, Ames MM, Goetz MP, Davis MA (2014) Comparative uterotrophic effects of endoxifen and tamoxifen in ovariectomized sprague-dawley rats. Toxicol Pathol. doi:10.1177/0192623314525688
Frankel C, Palmieri FM (2010) Lapatinib side-effect management. Clin J Oncol Nurs 14(2):223–233. doi:10.1188/10.CJON.223-233
Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H (2001) Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 276(13):9817–9824. doi:10.1074/jbc.M010840200
Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX (2009) Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15(5):429–440. doi:10.1016/j.ccr.2009.03.020
Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL (2002) Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 62(14):4132–4141
Acknowledgements
The authors would like to thank Drs. Gigi Ngar Chee Chiu, Julia M. W. Gee, Dawn Waterhouse and Yi Yan Yang for providing the cell lines used in this study.
Funding
This work was supported by the Singapore Ministry of Education (MOE) Tier 1 Grant (R-148-000-204-112) and National University of Singapore (NUS) Grant (C-148-000-003-001) provided to Eric Chun Yong Chan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chan, J.C.Y., Ong, P.S., Lim, P. et al. Synergistic disruption of ERα/HER2 crosstalk by endoxifen and lapatinib in breast cancer cells. Cancer Chemother Pharmacol 79, 117–130 (2017). https://doi.org/10.1007/s00280-016-3211-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3211-7