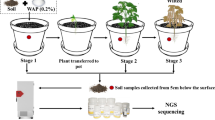
Abstract
The composition of the rhizosphere bacterial communities was compared among different maize cultivars by pyrosequencing. The cultivars were “Ye Dan 4,” “Ben Yu 9,” “Zheng Dan 958,” and “Li Min 33,” popularized in the 1980s, 1990s, 2000s, and 2010s, respectively, in Jilin Province, China. These cultivars harbored different bacterial dominant species. Significant differences were detected in the five dominant phyla, Actinobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, and Planctomycetes, especially between Li Min 33 and the three other cultivars. Li Min 33 had the lowest bacterial α-diversity, which was separated from other cultivars, according to a principal component analysis and the dissimilarity test of ADONIS. The γ-Proteobacteria, and within this, the genus Rhodanobacter, were significantly more abundant around Li Min 33 than around the other maize cultivars. The canonical correlation analysis indicated that the organic matter, soil pH, soil moisture, and leaf area index were important drivers of bacterial diversity. Mantel tests showed that the cultivar was significantly correlated with the microbial community composition. These results may aid in breeding or selecting new generations of plant cultivars that have the potential to support large populations of specific microbiota.





Similar content being viewed by others
References
Aira M, Gómez-Brandón M, Lazcano C, Bååth E, Dominguez J (2010) Plant genotype strongly modifies the structure and growth of maize rhizosphere microbial communities. Soil Biol Biochem 42:2276–2281
Ali B, Sabri AN, Ljung K, Hasnain S (2009) Auxin production by plant associated bacteria: impact on endogenous IAA content and growth of Triticum aestivum L. Appl Microbiol Lett 48:542–547
Amato KR, Yeoman CJ, Kent A, Righini N, Carbonero F, Estrada A, Gaskins HR, Stumpf RM, Yildirim S, Torralba M, Gillis M, Wilson BA, Nelson KE, White BA, Leigh SR (2013) Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J 7:1344–1353
An DS, Lee HG, Lee ST, Im WT (2009) Rhodanobacter ginsenosidimutans sp. nov., isolated from soil of a ginseng field in South Korea. Int J Syst Evol Microbiol 59:691–694
Araújo da Silva KR, Salles JF, Seldin L, van Elsas JD (2003) Application of a novel Paenibacillus-specific PCR-DGGE method and sequence analysis to assess the diversity of Paenibacillus spp. in the maize rhizosphere. J Microbiol Meth 54:213–231
Badri DV, Quintana N, El Kassis EG, Kim HK, Choi YH, Sugiyama A, Verpoorte R, Martinoia E, Manter DK, Vivanco JM (2009) An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol 151:2006–2017
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Baudoin E, Benizri E, Guckert A (2003) Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biol Biochem 35:1183–1192
Bell TH, Hassan SE, Lauron-Moreau A, Al-Otaibi F, Hijri M, Yergeau E, St-Arnaud M (2014) Linkage between bacterial and fungal rhizosphere communities in hydrocarbon-contaminated soils is related to plant phylogeny. ISME J 8:331–343
Bollmann A, Palumbo AV, Lewis K, Epstein SS (2010) Isolation and physiology of bacteria from contaminated subsurface sediments. Appl Environ Microbiol 76:7413–7419
Bradshaw JE, Dale MFB, Mackay GR (2003) Use of mid-parent values and progeny tests to increase the efficiency of potato breeding for combined processing quality and disease and pest resistance. Theor Appl Genet 107:36–42
Briones AM, Okabe S, Umemiya Y, Ramsing N, Reichardt W, Okuyama H (2002) Influence of different cultivars on populations of ammonia-oxidizing bacteria in the root environment of rice. Appl Environ Microbiol 68:3067–3075
Brusetti L, Francia P, Bertolini C, Pagliuca A, Borin S, Sorlini C, Abruzzese A, Sacchi G, Viti C, Giovannetti L, Giuntini E, Bazzicalupo M, Daffonchio D (2004) Bacterial communities associated with the rhizosphere of transgenic Bt 176 maize (Zea mays) and its non transgenic counterpart. Plant Soil 266:11–21
Bui TP, Kim YJ, Kim H, Yang DC (2010) Rhodanobacter soli sp. nov., isolated from soil of a ginseng field. Int J Syst Evol Microbiol 60:2935–2939
Castaldini M, Turrini A, Sbrana C, Benedetti A, Marchionni M, Mocali S, Fabiani A, Landi S, Santomassimo F, Pietrangeli B, Nuti MP, Miclaus N, Giovannetti M (2005) Impact of Bt corn on rhizospheric and soil eubacterial communities and on beneficial mycorrhizal symbiosis in experimental microcosms. Appl Environ Microbiol 71:6719–6729
Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M, Abad M, Kumar G, Salvador S, D’Ordine R, Navarro S, Back S, Fernandes M, Targolli J, Dasgupta S, Bonin C, Luethy MH, Heard JE (2008) Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol 147:446–455
Chamam A, Sanguin H, Bellvert F, Meiffren G, Comte G, Wisniewski-Dyé F, Bertrand C, Prigent-Combaret C (2013) Plant secondary metabolite profiling evidences strain dependent effect in the Azospirillum–Oryza sativa association. Phytochemistry 87:65–77
Chanway CP, Nelson LM, Holl FB (1988) Cultivar specific growth promotion of spring wheat Triticum aestivum L. by coexistent Bacillus species. Can J Microbiol 34:925–929
Corrales I, Amenós M, Poschenrieder C, Barceló J (2007) Phosphorus efficiency and root exudates in two contrasting tropical maize varieties. J Plant Nutr 30:887–900
Czarnota MA, Rimando AM, Weston LA (2003) Evaluation of root exudates of seven sorghum accessions. J Chem Ecol 29:2073–2083
De Clercq D, Van Trappen S, Cleenwerck I, Ceustermans A, Swings J, Coosemans J, Ryckeboer J (2006) Rhodanobacter spathiphylli sp. nov., a gammaproteobacterium isolated from the roots of Spathiphyllum plants grown in a compost-amended potting mix. Int J Syst Evol Microbiol 56:1755–1759
Den Herder G, Van Isterdael G, Beeckman T, De Smet I (2010) The roots of a new green revolution. Trends Plant Sci 15:600–607
Dohrmann AB, Küting M, Jünemann S, Jaenicke S, Schlüter A, Tebbe CC (2013) Importance of rare taxa for bacterial diversity in the rhizosphere of Bt- and conventional maize varieties. ISME J 7:37–49
Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V (2015) Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci U S A 112:E911–E920
Fan TW, Lane AN, Shenker M, Bartley JP, Crowley D, Higashi RM (2001) Comprehensive chemical profiling of gramineous plant root exudates using high-resolution NMR and MS. Phytochemistry 57:209–221
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Figueiredo MV, Burity HA, Martínez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182–188
Gahoonia TS, Nielsen NE (2004) Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 260:47–57
Germida JJ, Siciliano SD (2001) Taxonomic diversity of bacteria associated with the roots of modern, recent and ancient wheat cultivars. Biol Fert Soils 33:410–415
Grayston S, Campbell C (1996) Functional biodiversity of microbial communities in the rhizospheres of hybrid larch (Larix eurolepis) and Sitka spruce (Picea sitchensis). Tree Physiol 16:1031–1038
Haichar FE, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achouak W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230
Herbert Y, Guingo E, Loudet O (2001) The response of root/shoot partitioning and root morphology to light reduction in maize genotypes. Crop Sci 16:162–211
Hinsinger P, Plassard C, Tang C, Jaillard B, Tang CX (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Im WT, Lee ST, Yokota A (2004) Rhodanobacter fulvus sp. nov., a beta-galactosidase-producing gammaproteobacterium. J Gen Appl Microbiol 50:143–147
Innerebner G, Knief C, Vorholt JA (2011) Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl Environ Microbiol 77:3202–3210
King EB, Parke JL (1993) Biocontrol of Aphanomyces root rot and Pythium damping-off by Pseudomonas cepacia AMMD on four pea cultivars. Plant Dis 77:1185–1188
Kostka JE, Green SJ, Rishishwar L, Prakash O, Katz LS, Mariño-Ramíreze L, Jordana IK, Munk C, Ivanova N, Mikhailova N, Watson DB, Brown SD, Palumbo AV, Brooks SC (2012) Genome sequences for six Rhodanobacter strains, isolated from soils and the terrestrial subsurface, with variable denitrification capabilities. J Bacteriol 194:4461–4462
Kucey RMN (1988) Plant growth-altering effects of Azospirillum brasilense and Bacillus C-11-25 on two wheat cultivars. J Appl Bacteriol 64:187–196
Kumar PS, Brooker MR, Dowd SE, Camerlengo T (2011) Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS One 6:e20956
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Lee EA, Tollenaar M (2007) Physiological basis of successful breeding strategies for maize grain yield. Crop Sci 47:S202–S215
Leeman M, van Pelt JA, den Ouden FM, Heinsbroek M, Bakker PAHM, Schippers B (1995) Induction of systemic resistance by Pseudomonas fluorescens in radish cultivars differing in susceptibility to Fusarium wilt, using a novel bioassay. Eur J Plant Pathol 101:655–664
Li J (2013) The identification of density-tolerance and genetic study of maize under different density selection pressure. Ph.D Dissertation. Jinlin Agricultural University
Liu L, Kloepper JW, Tuzun S (1995) Induction of systemic resistance in cucumber by plant growth-promoting rhizobacteria: duration of protection and effect of host resistance on protection and root colonization. Phytopathology 85:1064–1068
Liu P, Lin Q, Sui F, Sun Z (1994) Study on the characteristics of root system in high-yield upright-leaf maize. J Maize Sciences 3:36–39
Mansfield BD, Mumm RH (2014) Survey of plant density tolerance in US maize germplasm. Crop Sci 54:157–173
Mansouri H, Petit A, Oger P, Dessaux Y (2002) Engineered rhizosphere: the trophic bias generated opine-producing plants is independent of the opine, the soil origin, and the plant species. Appl Environ Microbiol 68:2562–2566
Mazzola M, Funnell DL, Raaijmakers JM (2004) Wheat cultivar-specific selection of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas species from resident soil populations. Microb Ecol 48:338–348
Mazzola M, Gu Y-H (2000) Phyto-management of microbial community structure to enhance growth of apple in replant soils. Acta Hortic 532:73–78
Merbach W, Mirus E, Knof G, Remus R, Ruppel S, Russow R, Gransee A, Schulze J (1999) Release of carbon and nitrogen compounds by plant roots and their possible ecological importance. J Plant Nutr Soil Sc 162:373–383
Micallef SA, Channer S, Shiaris MP, Colon-Carmona A (2009) Plant age and genotype impact the progression of bacterial community succession in the Arabidopsis rhizosphere. Plant Signal Behav 4:777–780
Milling A, Smalla K, Xaver F, Maidl K, Schloter M, Munch JC (2004) Effects of transgenic potatoes with an altered starch composition on the diversity of soil and rhizosphere bacteria and fungi. Plant Soil 266:23–39
Nalin R, Simonet P, Vogel TM, Normand P (1999) Rhodanobacter lindaniclasticus gen. Nov., sp. nov., a lindane-degrading bacterium. Int J Syst Bacteriol 49:19–23
Notz R, Maurhofer M, Schnider-Keel U, Duffy B, Haas D, Défago G (2001) Biotic factors affecting expression of the 2,4-diacetylphloroglucinol biosynthesis gene phlA in Pseudomonas fluorescens biocontrol strain CHA0 in the rhizosphere. Phytopathology 91:873–881
Palacios OA, Bashan Y, de-Bashan LE (2014) Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—an overview. Biol Fert Soils 50:415–432
Pathan SI, Ceccherini MT, Hansen MA, Giagnoni L, Ascher J, Arenella M, Sørensen SJ, Pietramellara G, Nannipieri P, Renella G (2015a) Maize lines with different nitrogen use efficiency select bacterial communities with different β-glucosidase-encoding genes and glucosidase activity in the rhizosphere. Biol Fert Soils 51:995–1004
Pathan SI, Ceccherini MT, Pietramellara G, Puschenreiter M, Giagnoni L, Arenella M, Varanini Z, Nannpieri P, Renella G (2015b) Enzyme activity and microbial community structure in the rhizosphere of two maize lines differing in N use efficiency. Plant Soil 387:413–424
Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, Buckler ES, Ley RE (2013) Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci U S A 110:6548–6553
Picard C, Bosco M (2006) Heterozygosis drives maize hybrids to select elite 2,4-diacethylphloroglucinol-producing Pseudomonas strains among resident soil populations. FEMS Microbiol Ecol 58:193–204
Picard C, Bosco M (2005) Maize heterosis affects the structure and dynamics of indigenous rhizospheric auxins producing Pseudomonas populations. FEMS Microbiol Ecol 53:349–357
Picard C, Frascaroli E, Bosco M (2004) Frequency and biodiversity of 2,4-diacetylphloroglucinol-producing rhizobacteria are differentially affected by the genotype of two maize inbred lines and their hybrid. FEMS Microbiol Ecol 49:207–215
Raaijmakers JM, Vlami M, De Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81:537–547
R Core Team. R (2013) A language and environment for statistical computing R Foundation for Statistical Computing: Vienna, Austria. http://www.R-project.org/
Sangabriel-Conde W, Negrete-Yankelevich S, Maldonado-Mendoza IE, Trejo-Aguilar D (2014) Native maize landraces from Los Tuxtlas, Mexico show varying mycorrhizal dependency for P uptake. Biol Fert soils 50:405–414
Sanguin H, Sarniguet A, Gazengel K, Moёnne-Loccoz Y, Grundmann GL (2009) Rhizosphere bacterial communities associated with disease suppressiveness stages of take-all decline in wheat monoculture. New Phytol 184:694–707
Smith KP, Handelsman J, Goodman RM (1997) Modeling dose-response relationships in biocontrol: partitioning host responses to the pathogen and biocontrol agent. Phytopathology 87:720–729
Smith KP, Handelsman J, Goodman RM (1999) Genetic basis in plants for interactions with disease-suppressive bacteria. Proc Natl Acad Sci U S A 96:4786–4790
Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (1996) Methods of soil analysis. Part 3—chemical methods. Soil Science Society of America Inc, Madison, WI
Tollenaar M, Daynard TB (1978) Relationship between assimilate source and reproductive sink in maize grown in a short season environment. Agron J 70:219–223
Vakili NG (1992) Biological seed treatment of corn with mycopathogenic fungi. J Phytopathol 134:313–323
Vakili NG, Bailey TB Jr (1989) Yield response of corn hybrids and inbred lines to phylloplane treatment with mycopathogenic fungi. Crop Sci 29:183–190
van den Heuvel RN, van der Biezen E, Jetten MS, Hefting MM, Kartal B (2010) Denitrification at pH 4 by a soil-derived Rhodanobacter-dominated community. Enviro Microbiol 12:3264–3271
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wang L, An DS, Kim SG, Jin FX, Lee ST, Im WT (2011) Rhodanobacter panaciterrae sp. nov., a bacterium with ginsenoside-converting activity isolated from soil of a ginseng field. Int J Syst Evol Microbiol 61:3028–3032
Weinert N, Piceno Y, Ding GC, Meincke R, Heuer H, Berg G, Schloter M, Andersen G, Smalla K (2011) PhyloChip hybridization uncovered an enormous bacterial diversity in the rhizosphere of different potato cultivars: many common and few cultivar-dependent taxa. FEMS Microbiol Ecol 75:497–506
Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
Weon HY, Kim BY, Hong SB, Jeon YA, Kwon SW, Go SJ, Koo BS (2007) Rhodanobacter ginsengisoli sp. nov. and Rhodanobacter terrae sp. nov., isolated from soil cultivated with Korean ginseng. Int J Syst Evol Microbiol 57:2810–2813
Zhang H, Kim M, Sun Y, Dowd SE, Shi H, Paré PW (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant 21:737–744
Acknowledgements
This work was supported by the Special Fund for Agro-Scientific Research of the Ministry of Agriculture of China (201103001) and the National Key Research and Development Program of China (2016YFD0300203).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Xinya Wen and Meng Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wen, X., Wang, M., Ti, J. et al. Bacterial community composition in the rhizosphere of maize cultivars widely grown in different decades. Biol Fertil Soils 53, 221–229 (2017). https://doi.org/10.1007/s00374-016-1169-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-016-1169-6