Abstract
Background
hTFM in primary vulvar cancer is an important prognostic factor. Ideally, a diameter of > 8 mm should be achieved after primary surgery. The role of VIN III persistence after primary surgery in vulvar cancer is still unclear. The main objective of the current study was to study the role of residual VIN III re-excision and compare differences in disease-free survival among patients with different hTFM and in primary vulvar cancer.
Methods
Forty-two patients with residual adjacent VIN III after primary surgery for vulvar cancer which were operated between 2000 and 2016 in our clinic were enrolled in this retrospective study. Re-excision rates for residual adjacent VIN III were calculated. According to the histological margin patients were divided into three group: < 3, 3–8 and > 8 mm. Univariate and multivariate analyses were conducted using the Kaplan–Meier method and Cox proportional hazards models, respectively.
Results
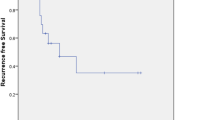
The vast majority of patients had pT1b stage (57.1%), grading G2 (71.4%) and lymph node-negative (45.3%) disease at first diagnosis. The re-excision rate was 57.1%. The 5-year disease-free survival (DFS) rates in patients with < 3, 3–8 and > 8 mm hTFM were 50.0, 50.0 and 81.0%, respectively (p = 0.032). The 5-year DFS rates in patients with re-excision and without re-excision for VIN III were 77.3 and 52.9%, respectively (p = 0.060). In univariate analysis was solely hTFM > 8 mm a prognostic factor for DFS (p = 0.017).
Conclusions
hTFM may be a potential prognostic indicator for DFS in vulvar cancer patients. Re-excision for residual adjacent VIN III could not be established as a prognostic factor for DFS after primary surgery in squamous cell cancer of vulva.

Similar content being viewed by others
References
Hampl M, Deckers-Figiel S, Hampl JA et al (2008) New aspects of vulvar cancer: changes in localization and age of onset. Gynecol Oncol 109(3):340–345
Al-Ghamdi A, Freedman D, Miller D et al (2002) Vulvar squamous cell carcinoma in young women: a clinicopathologic study of 21 cases. Gynecol Oncol 84(1):94–101
van Seters M, van Beurden M, de Craen AJM (2005) Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol 97(2):645–651
Reyes MC, Cooper K (2014) An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. J Clin Pathol 67(4):290–294
De Vuyst H, Clifford GM, Nascimento MC et al (2009) Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 124(7):1626–1636
Insinga RP, Liaw K-L, Johnson LG et al (2008) A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 17(7):1611–1622
Helm CW, Hatch K, Austin JM et al (1992) A matched comparison of single and triple incision techniques for the surgical treatment of carcinoma of the vulva. Gynecol Oncol 46(2):150–156
Raimond E, Pelissier A, Etienette Emeriau M et al (2017) Use of negative pressure wound therapy after vulvar carcinoma: case studies. J Wound Care 26(2):72–74
Likes WM, Stegbauer C, Tillmanns T et al (2007) Correlates of sexual function following vulvar excision. Gynecol Oncol 105(3):600–603
Grimm D, Eulenburg C, Brummer O et al (2016) Sexual activity and function after surgical treatment in patients with (pre)invasive vulvar lesions. Support Care Cancer 24(1):419–428
Heaps JM, Fu YS, Montz FJ et al (1990) Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol 38(3):309–314
Chan JK, Sugiyama V, Pham H et al (2007) Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol 104(3):636–641
Woelber L, Griebel L-F, Eulenburg C et al (1990) Role of tumour-free margin distance for loco-regional control in vulvar cancer—a subset analysis of the Arbeitsgemeinschaft Gynäkologische Onkologie CaRE-1 multicenter study. Eur J Cancer Oxf Engl 2016(69):180–188
Baiocchi G, Mantoan H, de Brot L et al (2015) How important is the pathological margin distance in vulvar cancer? Eur J Surg Oncol 41(12):1653–1658
Woolderink JM, de Bock GH, de Hullu JA et al (2006) Patterns and frequency of recurrences of squamous cell carcinoma of the vulva. Gynecol Oncol 103(1):293–299
Preti M, Ronco G, Ghiringhello B et al (2000) Recurrent squamous cell carcinoma of the vulva: clinicopathologic determinants identifying low risk patients. Cancer 88(8):1869–1876
Sobin L, Gospodarowicz M, Wittekind C (2011) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, PA
Oonk MHM, Planchamp F, Baldwin P et al (2017) European society of gynaecological oncology guidelines for the management of patients with vulvar cancer. Int J Gynecol Cancer 27(4):832–837
Woelber L, Kock L, Gieseking F et al (2011) Clinical management of primary vulvar cancer. Eur J Cancer 47(15):2315–2321
Nooij LS, van der Slot MA, Dekkers OM et al (1990) Tumour-free margins in vulvar squamous cell carcinoma: does distance really matter? Eur J Cancer Oxf Engl 2016(65):139–149
Dittmer C, Fischer D, Diedrich K et al (2012) Diagnosis and treatment options of vulvar cancer: a review. Arch Gynecol Obstet 285(1):183–193
Del Pino M, Rodriguez-Carunchio L, Ordi J (2013) Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology 62(1):161–175
Sideri M, Jones RW, Wilkinson EJ et al (2005) Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD vulvar oncology subcommittee. J Reprod Med 50(11):807–810
Edwards CL, Tortolero-Luna G, Linares AC et al (1996) Vulvar intraepithelial neoplasia and vulvar cancer. Obstet Gynecol Clin North Am 23(2):295–324
Groenen SMA, Timmers PJ, Burger CW (2010) Recurrence rate in vulvar carcinoma in relation to pathological margin distance. Int J Gynecol Cancer 20(5):869–873
Modesitt SC, Waters AB, Walton L et al (1998) Vulvar intraepithelial neoplasia III: occult cancer and the impact of margin status on recurrence. Obstet Gynecol 92(6):962–966
Stehman FB, Bundy BN, Ball H et al (1996) Sites of failure and times to failure in carcinoma of the vulva treated conservatively: a gynecologic oncology group study. Am J Obstet Gynecol 174(4):1128–1132 (discussion 1132–1133)
Te Grootenhuis NC, van der Zee AGJ, van Doorn HC et al (2016) Sentinel nodes in vulvar cancer: long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol Oncol 140(1):8–14
Author information
Authors and Affiliations
Contributions
KG: data collection and management, data analysis, statistical analysis (descriptive), manuscript writing and editing; MS: data collection and management, data analysis; IS: data collection; RW: histological examination; TK: data analysis, statistical analysis (correlation, survival); SB: project development; AE-B: protocol and project development, manuscript correction.
Corresponding author
Ethics declarations
Conflict of interest
No financial or personal conflict of interest by any of the authors to declare.
Rights and permissions
About this article
Cite this article
Gasimli, K., Straussner, M., Schmeil, I. et al. Impact of re-excision of residual adjacent vulvar intraepithelial neoplasia (VIN III) and histological tumour-free margin (hTFM) on survival in primary squamous cell carcinoma of vulva. Arch Gynecol Obstet 298, 945–950 (2018). https://doi.org/10.1007/s00404-018-4887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4887-1