Abstract
Purpose
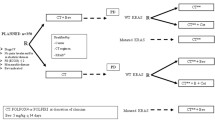
Uncertainty exists regarding comparative effectiveness of cetuximab versus bevacizumab in metastatic colorectal cancer (mCRC). We conducted a retrospective head-to-head multi-cohort study comparing clinical outcomes from both antibodies
Methods
Cohorts were defined by treatment line and subgroups by (K)RAS status and tumour sidedness. Among other outcomes, we estimated and compared response rates, progression-free (PFS) and overall survival (OS).
Results
Between January 2010 and April 2018, 311 patients were included. Except for (K)RAS mutation status, baseline characteristics were balanced across treatment groups. In the full analysis of first and second-line cohorts, PFS (first-line: HR = 0.85; 95% CI 0.64 to 1.13; P = 0.26; second-line: HR = 1.16; 95% CI 0.74 to 1.83; P = 0.51) and OS (first-line: HR = 0.83; 95% CI 0.61 to 1.15; P = 0.26; second-line: HR = 0.88; 95% CI 0.56 to 1.38; P = 0.58) were similar between bevacizumab and cetuximab arms. In subgroup analyses of first-line therapy, we found a survival difference favouring bevacizumab in right-sided tumours (PFS: HR = 0.52; 95% CI 0.29 to 0.93; P = 0.025; OS: HR = 0.60; 95% CI 0.32 to 1.12; P = 0.11), but not in left-sided (HR = 1.04; 95% CI 0.75 to 1.46; P = 0.81; OS: HR = 0.94; 95% CI 0.65 to 1.36; P = 0.74), or (K)RAS wild-type tumours (PFS: HR = 0.91; 95% CI 0.60 to 1.40; P = 0.67; OS: HR = 0.79; 95% CI 0.50 to 1.25; P = 0.31). Response rates were similar across treatment groups, except for the subgroup of patients bearing right-sided primaries, where bevacizumab performed substantially better.
Conclusion
This study provides evidence suggesting bevacizumab and cetuximab lead to similar effectiveness outcomes in mCRC, except for right-sided tumours, where cetuximab seemed to show considerably poorer outcomes. Further research is needed to confirm these results.





Similar content being viewed by others
References
Aderka D, Stintzing S, Heinemann V (2019) Explaining the unexplainable: discrepancies in results from the CALGB/SWOG 80405 and FIRE-3 studies. Lancet Oncol 20(5):e274–e283. https://doi.org/10.1016/S1470-2045(19)30172-X
Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V et al (2017) Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 28(8):1713–1729. https://doi.org/10.1093/annonc/mdx175
Bennouna J, Hiret S, Bertaut A, Bouché O, Deplanque G, Borel C, François E et al (2019) Continuation of bevacizumab vs cetuximab plus chemotherapy after first progression in KRAS wild-type metastatic colorectal cancer: the UNICANCER PRODIGE18 randomized clinical trial. JAMA Oncol 5(1):83–90. https://doi.org/10.1001/jamaoncol.2018.4465
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. https://doi.org/10.3322/caac.21492
Chibaudel B, Bonnetain F, Shi Q, Buyse M, Tournigand C, Sargent DJ, Allegra CJ, Goldberg RM, De Gramont A (2011) Alternative end points to evaluate a therapeutic strategy in advanced colorectal cancer: evaluation of progression-free survival, duration of disease control, and time to failure of strategy: an aide et recherche en Canceŕologie Digestive group study. J Clin Oncol 29(31):4199–4204. https://doi.org/10.1200/JCO.2011.35.5867
Choi KW, Hong SW, Chang YG, Lee WY, Lee B, Paik IW, Lee H (2014) Inflammation-based score (Glasgow Prognostic Score) as an independent prognostic factor in colorectal cancer patients. Ann Surg Treatment Res 86(6):309–313. https://doi.org/10.4174/astr.2014.86.6.309
Collett D (2003) Modelling survival data in medical research: texts in statistical science series, 2nd edn. Chapman & Hall/CRC, Boca Raton
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (Version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Elez E, Argilés G, Tabernero J (2015) First-line treatment of metastatic colorectal cancer: interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol 16(11):52. https://doi.org/10.1007/s11864-015-0369-x
Goey KKH, Sørbye H, Glimelius B, Adams RA, André T, Arnold D, Berlin JD et al (2018) Consensus statement on essential patient characteristics in systemic treatment trials for metastatic colorectal cancer: supported by the ARCAD group. Eur J Cancer 100:35–45. https://doi.org/10.1016/j.ejca.2018.05.010
Guinney J, Dienstmann R, Wang X, De Reyniès A, Schlicker A, Soneson C, Marisa L et al (2015) The consensus molecular subtypes of colorectal cancer. Nat Med 21(11):1350–1356. https://doi.org/10.1038/nm.3967
Heinemann V, Weikersthal LFV, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, Heintges T, Lerchenmüller C, Kahl C (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V (2017) The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 70:87–98. https://doi.org/10.1016/j.ejca.2016.10.007
Innocenti F, Fang-Shu O, Xueping Q, Zemla TJ, Niedzwiecki D, Tam R, Mahajan S et al (2019) Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. https://doi.org/10.1200/JCO.18.01798
Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U (2009) Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices task force report-part III. Value Health 12(8):1062–1073. https://doi.org/10.1111/j.1524-4733.2009.00602.x
Kassambara, A and M Kosinski (2018) Survminer: drawing survival curves using ‘Ggplot2’. R Package Version 0.4.3.
Lenz H-J, Fang-Shu Ou, Venook AP, Hochster HS, Niedzwiecki D (2019) Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: results from CALGB/SWOG 80405 (Alliance). J Clin Oncol. https://doi.org/10.1200/JCO.18.02258
Loupakis F, Hurwitz HI, Saltz L, Arnold D, Grothey A, Nguyen QL, Osborne S, Talbot J, Srock S, Lenz H-J (2018) Impact of primary tumour location on efficacy of bevacizumab plus chemotherapy in metastatic colorectal cancer. Br J Cancer 119(12):1451–1455. https://doi.org/10.1038/s41416-018-0304-6
Modest DP, Stintzing S, Von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E et al (2015) Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2015.61.2887
Rivera F, Meinolf Karthaus J, Hecht R, Sevilla I, Forget F, Fasola G, Canon JL, Guan X, Demonty G, Schwartzberg LS (2017) Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with MFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis 32(8):1179–1190. https://doi.org/10.1007/s00384-017-2800-1
Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE et al (2017) Molecular biomarkers for the evaluation of colorectal cancer: guideline from The American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol 35(13):1453–1496. https://doi.org/10.1200/JCO.2016.71.9807
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A (2017) Colorectal cancer statistics 2017. Cancer J Clin 67(3):177–193. https://doi.org/10.3322/caac.21395
Stintzing S, Modest DP, Rossius L, Lerch MM, Fischer L, von Weikersthal T, Decker AK et al (2016) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17(10):1426–1434. https://doi.org/10.1016/S1470-2045(16)30269-8
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the cox model. Statistics for Biology and Health, New York
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. https://doi.org/10.1093/annonc/mdw235
Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Fruth B, Meyerhardt JA, Schrag D et al (2017) Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer. JAMA 317(23):2392. https://doi.org/10.1001/jama.2017.7105
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Wang Z-X, Hao-Xiang Wu, He M-M, Wang Y-N, Luo H-Y, Ding P-R, Xie D et al (2019) Chemotherapy with or without anti-EGFR agents in left- and right-sided metastatic colorectal cancer: an updated meta-analysis. J Natl Compr Canc Netw 17(7):805–811. https://doi.org/10.6004/jnccn.2018.7279
Wille-Jørgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, Renehan AG, Horváth-Puho E, Påhlman L, Sørensen HT (2018) Effect of more vs less frequent follow-up testing on overall and colorectal cancer-specific mortality in patients with stage II or III colorectal cancer the COLOFOL randomized clinical trial. JAMA 319(20):2095–2103. https://doi.org/10.1001/jama.2018.5623
Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, Jayakumaran G et al (2018) Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 33(1):125–136.e3. https://doi.org/10.1016/j.ccell.2017.12.004
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RPM and APM conceived, designed and planned the study. RPM led the study assessments and data acquisition process. HLP and AQ provided expertise on metastatic colorectal cancer and contributed to data acquisition. RPM led the interpretation and discussion of the results and drafting of the manuscript. PH and ARG performed the statistical analysis, interpreted and discussed the results, and contributed to drafting of the manuscript. All authors read, provided feedback and approved the final manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Approved by local committee.
Transparency
All authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marques, R.P., Godinho, A.R., Heudtlass, P. et al. Cetuximab versus bevacizumab in metastatic colorectal cancer: a comparative effectiveness study. J Cancer Res Clin Oncol 146, 1321–1334 (2020). https://doi.org/10.1007/s00432-020-03167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03167-0