Abstract
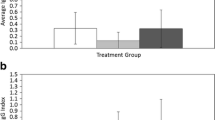
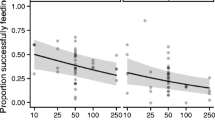
Immune function is an important component of host fitness, and high investment in immunity should occur when the benefits outweigh the costs, such as when risk of parasitism is high. We sampled two rodent hosts, white-footed mice (Peromyscus leucopus), and prairie voles (Microtus ochrogaster), and their tick, flea, and mite ectoparasites. A bacterial killing assay was used to measure the host’s innate immune function. We hypothesized that classes of hosts (species, sexes, or age classes) with overall higher tick burdens would have a higher innate immune function as an evolutionary response to historically greater exposure. We hypothesized a weaker relationship between the fleas and mites and immune function because of high host specificity in fleas and the absence of known vector function in North American mites. Ectoparasites were significantly overdispersed on hosts. In accordance with our hypothesis, Peromyscus that had higher tick burdens also exhibited significantly higher bacterial killing ability compared to Microtus. There was no significant difference in total flea burden between rodent species and no relationship with bacterial killing ability. Microtus had higher burdens of mites in each order than Peromyscus, and female rodents had higher mite burdens than males. The benefits of maintaining high levels of innate immune factors appear to be greater than the energetic costs for Peromyscus compared to Microtus.



Similar content being viewed by others
References
Abbot P, Aviles AE, Eller L, Durden LA (2007) Mixed infections, cryptic diversity, and vector-borne pathogens: evidence from Polygenis fleas and Bartonella species. Appl Environ Microbiol 73:6045–6052
Allen JR (1994) Host resistance to ectoparasites. Rev Sci Tech Off Int Épiz 13:1287–1303
Amerault TE, Rose JE, Kuttler KL (1981) Comparative titration of Anaplasma marginale antibodies by card agglutination and complement-fixation tests. Am J Vet Res 42:1055–1056
Anderson JF, Johnson RC, Magnarelli LA, Hyde FW, Myers JE (1986) Peromyscus leucopus and Microtus pennsylvanicus simultaneously infected with Borrelia burgdorferi and Babesia microti. J Clin Microbiol 23:135–137
Baucom RS, de Roode JC (2011) Ecological immunology and tolerance in plants and animals. Funct Ecol 25:18–28
Bordes F, Ponlet N, de Bellocq JG, Ribas A, Krasnov BR, Morand S (2012) Is there sex-biased resistance and tolerance in Mediterranean wood mouse (Apodemus sylvaticus) populations facing multiple helminth infections? Oecologia 170:123–135
Boughton RK, Joop G, Armitage SAO (2011) Outdoor immunology: methodological considerations for ecologists. Funct Ecol 25:81–100
Bradley JE, Jackson JA (2008) Measuring immune system variation to help understand host-pathogen community dynamics. Parasitology 135:807–823
Brinkerhoff RJ, Martin AP, Jones RT, Collinge SK (2011) Population genetic structure of the prairie dog flea and plague vector, Oropsylla hirsuta. Parasitology 138:71–79
Brown SJ (1988) Highlights of contemporary research on host immune-responses to ticks. Vet Parasitol 28:321–334
Burkot TR, Schneider BS, Pieniazek NJ, Happ CM, Rutherford JS, Slemenda SB, Hoffmeister E, Maupin GO, Zeidner NS (2000) Babesia microti and Borrelia bissetii transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology 121:595–599
Calabrese JM, Brunner JL, Ostfeld RS (2011) Partitioning the aggregation of parasites on hosts into intrinsic and extrinsic components via an extended poisson-gamma mixture model. PLoS One 6:e29215
Chester EM, Bonu T, Demas GE (2010) Social defeat differentially affects immune responses in Siberian hamsters (Phodopus sungorus). Physiol Behav 101:53–58
Demas GE, Chefer V, Talan MI, Nelson RJ (1997) Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6 J mice. Am J Physiol Regul Integr Comp Physiol 273:R1631–R1637
Durden LA (1992) Ectoparasites of sympatric meadow voles and white footed mice at Fort Detrick, Maryland. J Med Entomol 29:761–766
Erickson DL, Anderson NE, Cromar LM, Jolley A (2009) Bacterial communities associated with flea vectors of plague. J Med Entomol 46:1532–1536
Ezenwa VO, Ekernas LS, Creel S (2011) Unravelling complex associations between testosterone and parasite infection in the wild. Funct Ecol 26:123–133
Fenton A, Viney ME, Lello J (2010) Detecting interspecific macroparasite interactions from ecological data: patterns and process. Ecol Lett 13:606–615
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Folstad I, Nilssen AC, Halvorsen O, Andersen J (1989) Why do male reindeer (Rangifer-T tarandus) have higher abundance of 2nd and 3rd instar larvae of Hypoderma-tarandus tarandi Than Females. Oikos 55:87–92
Fornoni J, Nunez-Farfan J, Valverde PL, Rausher MD (2004) Evolution of mixed strategies of plant defense allocation against natural enemies. Evolution 58:1685–1695
Francis DH, Buening GM, Amerault TE (1980) Characterization of immune-responses of cattle to erythrocyte stroma, Anaplasma antigen, and dodecanoic acid-conjugated Anaplasma antigen: humoral immunity. Am J Vet Res 41:362–367
Fuxjager MJ, Foufopoulos J, Diaz-Uriarte R, Marler CA (2011) Functionally opposing effects of testosterone on two different types of parasite: implications for the immunocompetence handicap hypothesis. Funct Ecol 25:132–138
Gasparini J, McCoy KD, Haussy C, Tveraa T, Boulinier T (2001) Induced maternal response to the Lyme disease spirochaete Borrelia burgdorferi sensu lato in a colonial seabird, the kittiwake Rissa tridactyla. Proc R Soc Lond B Biol Sci 268:647–650
Ginsberg HS (2008) Potential effects of mixed infections in ticks on transmission dynamics of pathogens: comparative analysis of published records. Exp Appl Acarol 46:29–41
Gomez-Diaz E, Gonzalez-Solis J, Peinado MA, Page RDM (2007) Lack of host-dependent genetic structure in ectoparasites of Calonectris shearwaters. Mol Ecol 16:5204–5215
Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH (2010) Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science 330:662–665
Graham AL, Shuker DM, Pollitt LC, Auld SKJR, Wilson AJ, Little TJ (2011) Fitness consequences of immune responses: strengthening the empirical framework for ecoimmunology. Funct Ecol 25:5–17
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds—a role for parasites. Science 218:384–387
Hawlena H, Rynkiewicz E, Toh E, Alfred A, Durden LA, Hastriter MW, Nelson DE, Rong R, Munro D, Dong Q, Fuqua C, Clay K (2013) The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J 7:221–223
Hawley DM, Altizer SM (2011) Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol 25:48–60
Hofmeister EK, Kolbert CP, Abdulkarim AS, Magera JMH, Hopkins MK, Uhl JR, Ambyaye A, Telford SR III, Cockerill FR III, Persing DH (1998) Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis 177:409–416
Iwasa M, Kasuya S, Noda N, Hioki A, Ito A, Ohtomo H (1990) Trombiculid mites (Acari: Trombiculidae) and Rickettsia tsutsugamushi isolated from wild rodents in a new endemic area of Japan. J Med Entomol 27:501–508
Janeway CA, Travers P, Walport M, Shlomchik M (2007) Immunobiology, 7th edn. Garland Publishing, New York
Jones PH, Britten HB (2010) The absence of concordant population genetic structure in the black-tailed prairie dog and the flea, Oropsylla hirsuta, with implications for the spread of Yersinia pestis. Mol Ecol 19:2038–2049
Keil D, Luebke RW, Pruett SB (2001) Quantifying the relationship between multiple immunological parameters and host resistance: probing the limits of reductionism. J Immunol 167:4543–4552
Klompen JSH, Black WCV, Keirans JE, Oliver JH Jr (1996) Evolution of ticks. Annu Rev Entomol 41:141–161
Krasnov BR, Shenbrot GI, Khokhlova IS, Hawlena H, Degen AA (2006) Temporal variation in parasite infestation of a host individual: does a parasite-free host remain uninfested permanently? Parasitol Res 99:541–545
Krebs CJ (1970) Microtus population biology—behavioral changes associated with population cycle in M. ochrogaster and M. pennsylvanicus. Ecology 51:34–52
Lindstrom KM, Foufopoulos J, Parn H, Wikelski M (2004) Immunological investments reflect parasite abundance in island populations of Darwin’s finches. Proc R Soc Lond B Biol Sci 271:1513–1519
LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F (2003) The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. PNAS 100:567–571
Martin LB, Weil ZM, Nelson RJ (2007) Immune defense and reproductive pace of life in Peromyscus mice. Ecology 88:2516–2528
McAloon FM, Durden LA (2000) Attachment sites and frequency distribution of erythraeid mites, Leptus indianensis (Acari: Prostigmata), ectoparasitic on harvestmen, Leiobunum formosum (Opiliones). Exp Appl Acarol 24:561–567
Millet S, Bennett J, Lee KA, Hau M, Klasing KC (2007) Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol 31:188–201
Muehlenbein MP (2006) Intestinal parasite infections and fecal steroid levels in wild chimpanzees. Am J Phys Anthropol 130:546–550
Mumford RE, Whitaker JOJ (1982) Mammals of Indiana. Indiana University Press, Bloomington
Nelson RJ, Demas GE (1996) Seasonal changes in immune function. Q Rev Biol 71:511–548
Ostfeld RS, LoGiudice K (2003) Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology 84:1421–1427
Ostfeld RS, Cepeda OM, Hazler KR, Miller MC (1995) Ecology of Lyme disease: habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecol Appl 5:353–361
Pedersen AB, Fenton A (2007) Emphasizing the ecology in parasite community ecology. Trends Ecol Evol 22:133–139
Perkins SE, Ferrari MF, Hudson PJ (2008) The effects of social structure and sex-biased transmission on macroparasite infection. Parasitology 135:1561–1569
Petney TN, Vanark H, Spickett AM (1990) On sampling tick populations: the problem of overdispersion. Onderstepoort J Vet Res 57:123–127
Puustinen S, Koskela T, Mutikainen P (2004) Direct and ecological costs of resistance and tolerance in the stinging nettle. Oecologia 139:76–82
Råberg L, Sim D, Read AF (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318:812–814
Radovsky FJ (1985) Evolution of mammalian mesostigmate mites. In: Kim KC (ed) Coevolution of parasitic arthropods and mammals. Wiley, New York
Roberts ML, Buchanan KL, Evans MR (2004) Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav 68:227–239
Saino N, Moller AP, Bolzern AM (1995) Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): an experimental test of the immunocompetence hypothesis. Behav Ecol 6:397–404
Schwanz LE, Voordouw MJ, Brisson D, Ostfeld RS (2011) Borrelia burgdorferi has minimal impact on the Lyme disease reservoir host Peromyscus leucopus. Vector-Borne Zoon Dis 11:117–124
Shaw DJ, Dobson AP (1995) Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111:S111–S133
Shaw DJ, Grenfell BT, Dobson AP (1998) Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117:597–610
Tieleman BI, Williams JB, Ricklefs RE, Klasing KC (2005) Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc Royal Soc B Biol Sci 272:1715–1720
Tilly K, Rosa PA, Stewart PE (2008) Biology of infection with Borrelia burgdorferi. Infect Dis Clin N Am 22:217–234
Traub R (1985) Coevolution of fleas and mammals. In: Kim KC (ed) Coevolution of parasitic arthropods and mammals. Wiley, New York, pp 295–437
Whitaker JO (1982) Ectoparasites of mammals of Indiana. Indiana Academy of Science, Indianapolis
Whitaker JO Jr., Walters BL, Castor LK, Ritzi CM, Wilson N (2007) Host and distribution lists of mites (Acari), parasitic and phoretic, in the hair or on the skin of North American wild mammals north of Mexico: records since 1974. Faculty Publications from the Harold W Manter Laboratory of Parasitology, University of Nebraska, Lincoln 1:1–173
Wikel SK (1999) Tick modulation of host immunity: an important factor in pathogen transmission. Int J Parasitol 29:851–859
Woolhouse MEJ, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JLK, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM (1997) Heterogeneities in the transmission of infectious agents: implications for the design of control programs. PNAS 94:338–342
Zavala-Castro JE, Zavala-Velazquez JE, Peniche-Lara GF, Sulu Uicab JE (2009) Human rickettsialpox, Southeastern Mexico. Emerg Infect Dis 15:1665–1667
Zuk M, Johnson K, Thornhill R, Ligon JD (1990a) Parasites and male ornaments in free-ranging and captive red jungle fowl. Behaviour 114:232–248
Zuk M, Thornhill R, Ligon JD, Johnson K (1990b) Parasites and mate choice in red jungle fowl. Am Zool 30:235–244
Zysling DA, Garst AD, Demas GE (2009) Photoperiod and food restriction differentially affect reproductive and immune responses in Siberian hamsters Phodopus sungorus. Funct Ecol 23:1–10
Acknowledgments
The authors thank J. Kagemann, A. Avalos, and W. Snydermann for their help with rodent trapping and ectoparasite collection, and Dr. J. Whitaker and Dr. R. Pinger for ectoparasite identification. We thank Dr. E. Chester and Dr. M. Ruíz for their guidance in optimizing the bacterial killing assay and for helpful discussions of the results. Funding was provided through an Indiana Academy of Science Senior Research Grant and an NSF Doctoral Dissertation Improvement Grant (award no. 1110514) to ER, NSF-NIH grant DEB-03268742 to KC, an Indiana Metabolomics and Cytomics Initiative of Indiana University (IU) grant awarded to KC, and an IU Center for Research in Environmental Sciences grant awarded to KC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rynkiewicz, E.C., Hawlena, H., Durden, L.A. et al. Associations between innate immune function and ectoparasites in wild rodent hosts. Parasitol Res 112, 1763–1770 (2013). https://doi.org/10.1007/s00436-013-3335-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3335-1