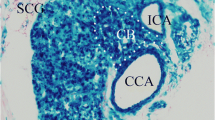
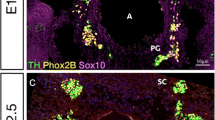
Abstract
Neuronal and neuroendocrine cells possess the capacity for Ca2+-regulated discharge of messenger molecules, which they release into synapses or the blood stream, respectively. The neural-crest-derived sympathoadrenal lineage gives rise to the sympathetic neurons of the autonomic nervous system and the neuroendocrine chromaffin cells of the adrenal medulla. These cells provide an excellent model system for studying common and distinct developmental mechanisms underlying the acquisition of neuroendocrine and neuronal properties. As catecholaminergic cells, they possess common markers related to noradrenaline synthesis, storage and release, but they also display diverging gene expression patterns and are morphologically and functionally different. The precise mechanisms that underlie the diversification of sympathoadrenal cells into neurons and neuroendocrine cells are not fully understood. However, in the past we could show that the establishment of a chromaffin phenotype does not depend on signals from the adrenal cortex and that chromaffin cells and sympathetic neurons apparently differ from the onset of their catecholaminergic differentiation. Nevertheless, the cues that specifically induce neuroendocrine features remain elusive. The early development of the progenitors of chromaffin cells and sympathetic neurons depends on a common set of transcription factors with overlapping but distinct influences on their development. In addition to the well-defined role of transcription factors as developmental regulators, our understanding of post-transcriptional gene regulation by microRNAs has substantially increased within the last few decades. This review highlights the major similarities and differences between chromaffin cells and sympathetic neurons, summarizes our current knowledge of the roles of selected transcription factors, microRNAs and environmental signals for the neuroendocrine differentiation of sympathoadrenal cells, and draws comparisons with the development of other endocrine and neuronal cells.

Similar content being viewed by others
References
Abdelmohsen K, Hutchison ER, Lee EK et al (2010) miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol Cell Biol 30:4197–4210
Åkerblom M, Sachdeva R, Barde I et al (2012) MicroRNA-124 is a subventricular zone neuronal fate determinant. J Neurosci 32:8879–8889
Anderson DJ (1993) Cell fate determination in the peripheral nervous system: the sympathoadrenal progenitor. J Neurobiol 24:185–198
Anderson DJ, Axel R (1985) Molecular probes for the development and plasticity of neural crest derivatives. Cell 42:649–662
Anderson DJ, Carnahan JF, Michelsohn A, Patterson PH (1991) Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J Neurosci 11:3507–3519
Anderson KD, Sengupta J, Morin M, Neve RL, Valenzuela CF, Perrone-Bizzozero NI (2001) Overexpression of HuD accelerates neurite outgrowth and increases GAP-43 mRNA expression in cortical neurons and retinoic acid-induced embryonic stem cells in vitro. Exp Neurol 168:250-258
Arda HE, Benitez CM, Kim SK (2013) Gene regulatory networks governing pancreas development. Dev Cell 25:5–13
Bernstein E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366
Bohn MC, Goldstein M, Black IB (1981) Role of glucocorticoids in expression of the adrenergic phenotype in rat embryonic adrenal gland. Dev Biol 82:1–10
Britsch S, Goerich DE, Riethmacher D et al (2001) The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 15:66–78
Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S (2003) Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5:877–889
Casarosa S, Fode C, Guillemot F (1999) Mash1 regulates neurogenesis in the ventral telencephalon. Development 126:525–534
Castro DS, Skowronska-Krawczyk D, Armant O et al (2006) Proneural bHLH and Brn proteins coregulate a neurogenic program through cooperative binding to a conserved DNA motif. Dev Cell 11:831–844
Dauger S, Pattyn A, Lofaso F et al (2003) Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 130:6635–6642
De Guevara ACL, Burigana F, Nicolini A, Lugnani F (2013) Neuroendocrine tumors of the lung: hystological classification, diagnosis, traditional and new therapeutic approaches. Curr Med Chem (in press)
Douarin NL, Kalcheim C (1999) The neural crest. Cambridge University Press, Cambridge
Duggan A, Madathany T, de Castro SCP et al (2008) Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J Comp Neurol 507:1497–1520
Elshatory Y, Everhart D, Deng M et al (2007) Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci 27:12707–12720
Ernsberger U, Esposito L, Partimo S et al (2005) Expression of neuronal markers suggests heterogeneity of chick sympathoadrenal cells prior to invasion of the adrenal anlagen. Cell Tissue Res 319:1–13
Falix FA, Tjon-A-Loi MRS, Gaemers IC et al (2013) DLK1 protein expression during mouse development provides new insights into its function. ISRN Dev Biol. doi:10.1155/2013/628962
Finotto S, Krieglstein K, Schober A et al (1999) Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development 126:2935–2944
Geppert M, Goda Y, Hammer RE et al (1994) Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79:717–727
Gierl MS, Karoulias N, Wende H et al (2006) The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev 20:2465–2478
Guillemot F, Joyner AL (1993) Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev 42:171–185
Guillemot F, Lo LC, Johnson JE et al (1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75:463–476
Gustavsson N, Lao Y, Maximov A et al (2008) Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A 105:3992–3997
Gustavsson N, Wei S-H, Hoang DN et al (2009) Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+−induced glucagon exocytosis in pancreas. J Physiol (Lond) 587:1169–1178
Gut P, Huber K, Lohr J et al (2005) Lack of an adrenal cortex in Sf1 mutant mice is compatible with the generation and differentiation of chromaffin cells. Development 132:4611–4619
Hendershot TJ, Liu H, Clouthier DE et al (2008) Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev Biol 319:179–191
Hirsch MR, Tiveron MC, Guillemot F et al (1998) Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 125:599–608
Hong SJ, Huh YH, Leung A, Choi HJ, Ding Y, Kang UJ, Yoo SH, Buettner R, Kim KS (2011) Transcription factor AP-2β regulates the neurotransmitter phenotype and maturation of chromaffin cells. Mol Cell Neurosci 46:245–251
Hou XE, Dahlström A (1996) Synaptic vesicle proteins in cells of the sympathoadrenal lineage. J Auton Nerv Syst 61:301–312
Howard MJ, Stanke M, Schneider C et al (2000) The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development 127:4073–4081
Huang T, Liu Y, Huang M et al (2010) Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J Mol Cell Biol 2:152–163
Huber K (2006) The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol 298:335–343
Huber K, Brühl B, Guillemot F et al (2002) Development of chromaffin cells depends on MASH1 function. Development 129:4729–4738
Huber K, Karch N, Ernsberger U et al (2005) The role of Phox2B in chromaffin cell development. Dev Biol 279:501–508
Huber K, Kalcheim C, Unsicker K (2009) The development of the chromaffin cell lineage from the neural crest. Auton Neurosci Basic Clin 151:10–16
Huber K, Narasimhan P, Shtukmaster S et al (2013) The LIM-homeodomain transcription factor Islet-1 is required for the development of sympathetic neurons and adrenal chromaffin cells. Dev Biol 380:286–298
Ito T, Udaka N, Ikeda M et al (2001) Significance of proneural basic helix-loop-helix transcription factors in neuroendocrine differentiation of fetal lung epithelial cells and lung carcinoma cells. Histol Histopathol 16:335–343
Kameda Y, Nishimaki T, Miura M et al (2007) Mash1 regulates the development of C cells in mouse thyroid glands. Dev Dyn 236:262–270
Kanji MS, Martin MG, Bhushan A (2013) Dicer1 is required to repress neuronal fate during endocrine cell maturation. Diabetes 62:1602–1611
Kloosterman WP, Lagendijk AK, Ketting RF et al (2007) Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol 5:e203
Laborda J (2000) The role of the epidermal growth factor-like protein dlk in cell differentiation. Histol Histopathol 15:119–129
Laborda J, Sausville EA, Hoffman T, Notario V (1993) dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem 268:3817–3820
Lammert E, Cleaver O, Melton D (2001) Induction of pancreatic differentiation by signals from blood vessels. Science 294:564–567. doi:10.1126/science.1064344
Langley K, Grant NJ (1999) Molecular markers of sympathoadrenal cells. Cell Tissue Res 298:185–206
Larsen JB, Jensen CH, Schrøder HD et al (1996) Fetal antigen 1 and growth hormone in pituitary somatotroph cells. Lancet 347:191
Lee YL, Helman L, Hoffman T, Laborda J (1995) dlk, pG2 and Pref-1 mRNAs encode similar proteins belonging to the EGF-like superfamily. Identification of polymorphic variants of this RNA. Biochim Biophys Acta 1261:223–232
Liang X, Song M-R, Xu Z et al (2011) Isl1 is required for multiple aspects of motor neuron development. Mol Cell Neurosci 47:215–222
Lloyd RV, Cano M, Rosa P et al (1988) Distribution of chromogranin A and secretogranin I (chromogranin B) in neuroendocrine cells and tumors. Am J Pathol 130:296–304
Loring JF, Erickson CA (1987) Neural crest cell migratory pathways in the trunk of the chick embryo. Dev Biol 121:220–236
Makeyev EV, Zhang J, Carrasco MA, Maniatis T (2007) The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27:435–448
Matsumoto S, Tanaka K, Yamamoto A et al (1987) Immunoelectron microscopic localization of dopamine beta-hydroxylase and chromogranin A in adrenomedullary chromaffin cells. Cell Struct Funct 12:483–496
McManus MT, Sharp PA (2002) Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 3:737–747
Mizrachi Y, Naranjo JR, Levi BZ, Pollard HB, Lelkes PI (1990) PC12 cells differentiate into chromaffin cell-like phenotype in coculture with adrenal medullary endothelial cells. Proc Natl Acad Sci U S A 87:6161-6165
Moriguchi T, Takako N, Hamada M et al (2006) Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 133:3871–3881
Morikawa Y, D’Autréaux F, Gershon MD, Cserjesi P (2007) Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol 307:114–126
Murakami T, Oukouchi H, Uno Y et al (1989) Blood vascular beds of rat adrenal and accessory adrenal glands, with special reference to the corticomedullary portal system: a further scanning electron microscopic study of corrosion casts and tissue specimens. Arch Histol Cytol 52:461–476
Neuman B, Wiedermann CJ, Fischer-Colbrie R et al (1984) Biochemical and functional properties of large and small dense-core vesicles in sympathetic nerves of rat and ox vas deferens. Neuroscience 13:921–931
Nishikawa E, Osada H, Okazaki Y et al (2011) miR-375 is activated by ASH1 and inhibits YAP1 in a lineage-dependent manner in lung cancer. Cancer Res 71:6165–6173
Ogawa M, Ishikawa T, Ohta H (1986) Transdifferentiation of endocrine chromaffin cells into neuronal cells. Curr Top Dev Biol 20:99–110
Parras CM, Schuurmans C, Scardigli R et al (2002) Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev 16:324–338
Pattyn A, Morin X, Cremer H et al (1999) The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399:366–370. doi:10.1038/20700
Pattyn A, Guillemot F, Brunet J-F (2006) Delays in neuronal differentiation in Mash1/Ascl1 mutants. Dev Biol 295:67–75
Pfaff SL, Mendelsohn M, Stewart CL et al (1996) Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84:309–320
Pogoda H-M, von der Hardt S, Herzog W et al (2006) The proneural gene ascl1a is required for endocrine differentiation and cell survival in the zebrafish adenohypophysis. Development 133:1079–1089
Potzner MR, Tsarovina K, Binder E et al (2010) Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development 137:775–784
Poy MN, Eliasson L, Krutzfeldt J et al (2004) A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432:226–230
Poy MN, Spranger M, Stoffel M (2007) microRNAs and the regulation of glucose and lipid metabolism. Diabetes Obes Metab 9 (Suppl 2):67–73
Poy MN, Hausser J, Trajkovski M et al (2009) miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A 106:5813–5818
Reiprich S, Stolt CC, Schreiner S et al (2008) SoxE proteins are differentially required in mouse adrenal gland development. Mol Biol Cell 19:1575–1586
Reissmann E, Ernsberger U, Francis-West PH et al (1996) Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development 122:2079–2088
Rohrer H (2011) Transcriptional control of differentiation and neurogenesis in autonomic ganglia. Eur J Neurosci 34:1563–1573
Rosenbaum JN, Duggan A, Garcia-Anoveros J (2011) Insm1 promotes the transition of olfactory progenitors from apical and proliferative to basal, terminally dividing and neuronogenic. Neural Dev 6:6
Ryan US, Ryan JW, Smith DS, Winkler H (1975) Fenestrated endothelium of the adrenal gland: freeze-fracture studies. Tissue Cell 7:181–190
Schmidt M, Huber L, Majdazari A et al (2011) The transcription factors AP-2β and AP-2α are required for survival of sympathetic progenitors and differentiated sympathetic neurons. Dev Biol 355:89–100
Schneider C, Wicht H, Enderich J et al (1999) Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron 24:861–870
Schober A, Unsicker K (2001) Growth and neurotrophic factors regulating development and maintenance of sympathetic preganglionic neurons. Int Rev Cytol 205:37–76
Schonn J-S, Maximov A, Lao Y et al (2008) Synaptotagmin-1 and −7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci U S A 105:3998–4003
Schwarz Q, Maden CH, Vieira JM, Ruhrberg C (2009) Neuropilin 1 signaling guides neural crest cells to coordinate pathway choice with cell specification. Proc Natl Acad Sci U S A 106:6164–6169
Shtukmaster S, Schier MC, Huber K et al (2013) Sympathetic neurons and chromaffin cells share a common progenitor in the neural crest in vivo. Neural Dev 8:12
Stubbusch J, Narasimhan P, Huber K et al (2013) Synaptic protein and pan-neuronal gene expression and their regulation by Dicer-dependent mechanisms differ between neurons and neuroendocrine cells. Neural Dev 8:16
Sun Y, Dykes IM, Liang X et al (2008) A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat Neurosci 11:1283–1293. doi:10.1038/nn.2209
Takeyama N, Ano Y, Wu G et al (2009) Localization of insulinoma associated protein 2, IA-2 in mouse neuroendocrine tissues using two novel monoclonal antibodies. Life Sci 84:678–687
Tornehave D, Jensen CH, Teisner B, Larsson LI (1996) FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol 106:535–542
Tsarovina K, Reiff T, Stubbusch J et al (2010) The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J Neurosci 30:10833–10843
Unsicker K, Krisch B, Otten U, Thoenen H (1978) Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: impairment by glucocorticoids. Proc Natl Acad Sci U S A 75:3498–3502
Visvanathan J, Lee S, Lee B et al (2007) The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev 21:744–749
Vogel KS, Weston JA (1990) The sympathoadrenal lineage in avian embryos. I. Adrenal chromaffin cells lose neuronal traits during embryogenesis. Dev Biol 139:1–12
Weihe E, Schäfer MK, Erickson JD, Eiden LE (1994) Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Mol Neurosci 5:149–164. doi:10.1007/BF02736730
Welcker JE, Hernandez-Miranda LR, Paul FE et al (2013) Insm1 controls development of pituitary endocrine cells and requires a SNAG domain for function and for recruitment of histone-modifying factors. Development 140:4947–4958
Wildner H, Gierl MS, Strehle M et al (2008) Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development 135:473–481
Wilson ME, Yang KY, Kalousova A et al (2005) The HMG box transcription factor Sox4 contributes to the development of the endocrine pancreas. Diabetes 54:3402–3409
Winkler H, Westhead E (1980) The molecular organization of adrenal chromaffin granules. Neuroscience 5:1803–1823
Wurtman RJ, Axelrod J (1966) Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem 241:2301–2305
Yu J-Y, Chung K-H, Deo M et al (2008) MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res 314:2618–2633
Zehir A, Hua LL, Maska EL et al (2010) Dicer is required for survival of differentiating neural crest cells. Dev Biol 340:459–467
Zhang N, Lin J, Chen J et al (2013a) MicroRNA 375 mediates the signaling pathway of corticotropin-releasing factor (CRF) regulating pro-opiomelanocortin (POMC) expression by targeting mitogen-activated protein kinase 8. J Biol Chem 288:10361–10373
Zhang Z-W, Men T, Feng R-C et al (2013b) miR-375 inhibits proliferation of mouse pancreatic progenitor cells by targeting YAP1. Cell Physiol Biochem 32:1808–1817
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huber, K. Segregation of neuronal and neuroendocrine differentiation in the sympathoadrenal lineage. Cell Tissue Res 359, 333–341 (2015). https://doi.org/10.1007/s00441-014-1947-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1947-0