Abstract
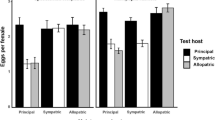
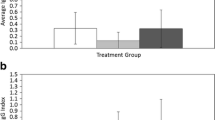
Mechanisms that cause nonrandom patterns of parasite distribution among host individuals may influence the population and evolutionary dynamics of both parasites and hosts, but are still poorly understood. We studied whether survival, reproduction, and behavioral responses of fleas (Xenopsylla conformis) changed with the age of their rodent hosts (Meriones crassus), experimentally disentangling two possible mechanisms: (a) differential survival and/or fitness reward of parasites due to host age, and (b) active parasite choice of a host of a particular age. To explore the first mechanism, we raised fleas on rodents of two age groups and assessed flea survival as well as the quantity and quality of their offspring. To explore the second mechanism, three groups of fleas that differed in their previous feeding experience (no experience, experience on juvenile or experience on adult rodents) were given an opportunity to choose between juvenile and adult rodents in a Y-maze. Fleas raised on juvenile rodents had higher survival and had more offspring that emerged earlier than fleas raised on adults. However, fleas did not show any innate preference for juvenile rodents, nor were they able to learn to choose them. In contrast to our predictions, based on a single previous exposure, fleas learned to choose adult rodents. The results suggest that two mechanisms—differential survival and fitness reward of fleas, and associative learning by them—affect patterns of flea distribution between juvenile and adult rodents. The former increases whereas the latter reduces flea densities on juvenile rodents. The ability of fleas to learn to choose adult but not juvenile hosts may be due to: (a) a stronger stimulus from adults, (b) a higher profitability of adults in terms of predictability and abundance, or (c) the evolutionary importance of recognizing adult but not juvenile hosts as representatives of the species.



Similar content being viewed by others
References
Alexander JOD (1986) The physiology of itch. Parasitol Today 2:345–351
Anderson RM, May RM (1978) Regulation and stability of host-parasite population interactions. I. Regulatory processes. J Anim Ecol 47:219–247
Atkinson RL, Atkinson RC, Smith EE, Bem DJ (1990) Introduction to psychology. Harcourt Brace Jovanovich, San Diego, CA
Castellano S, Cermelli P (2006) Reconciling sexual selection to species recognition: a process-based model of mating decision. J Theor Biol 242:529–538
Crompton DWT (1987) Host diet as a determinant of parasite growth, reproduction and survival. Mammal Rev 17:117–126
Davies CR, Gavgani ASM (1999) Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology 119:247–257
Djeridane Y (2002) Integumentary odoriferous glands in Meriones libycus: a histological study and related behavior. Folia Zool 51:37–58
Dukas R (1998) Evolutionary ecology of learning. In: Dukas R (ed) Cognitive ecology. University of Chicago Press, Chicago, IL, pp 129–174
Dupuy F, Sandoz JC, Giurfa M, Josens R (2006) Individual olfactory learning in camponotus ants. Anim Behav 72:1081–1091
Fichet-Calvet E, Wang JF, Jomaa I, Ismail RB, Ashford RW (2003) Patterns of the tapeworm Raillietina trapezoides infection in the fat sand rat Psammomys obesus in Tunisia: season, climatic conditions, host age and crowding effects. Parasitology 126:481–492
Gould JL (1993) Ethological and comparative perspective on honey bee learning. In: Papaj DR, Lewis AC (eds) Insect learning: ecological and evolutionary perspectives. Chapman and Hall, London, pp 18–50
Gregory RD, Montgomery SSJ, Montgomery WI (1992) Population biology of Heligmosomoides polygyrus (Nematoda) in the wood mouse. J Anim Ecol 61:749–757
Hawlena H, Abramsky Z, Krasnov BR (2005) Age-biased parasitism and density-dependent distribution of fleas (Siphonaptera) on a desert rodent. Oecologia 146:200–208
Hawlena H, Abramsky Z, Krasnov BR (2006a) Ectoparasites and age-dependent survival in a desert rodent. Oecologia 148:30–39
Hawlena H, Khokhlova IS, Abramsky Z, Krasnov BR (2006b) Age, intensity of infestation by flea parasites and body mass loss in a rodent host. Parasitology 133:187–193
Hawlena H, Bashary D, Abramsky Z, Krasnov BR (2007) Benefits, costs and constraints of anti-parasitic grooming in adult and juvenile rodents. Ethology 113:394–402
Henricson J (1977) The abundance and distribution of Diphyllobothrium dentriticum (Nitzsch) and D. ditremum (Creplin) in the char Salvelinus alpinus. J Fish Biol 11:231–248
Hinkle NC, Koehler PG, Patterson RS (1998) Host grooming efficiency for regulation of cat flea (Siphonaptera : Pulicidae) populations. J Med Entomol 35:266–269
Hudson PJ, Dobson AP (1997) Host-parasite processes and demographic consequences. In: Clayton DH, Moore J (eds) Host–parasite evolution. General principles and avian models, vol 7. Oxford University Press, New York, pp 128–154
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Karvonen A, Hudson PJ, Seppala O, Valtonen ET (2004) Transmission dynamics of a trematode parasite: exposure, acquired resistance and parasite aggregation. Parasitol Res 92:183–188
Kelly DW, Thompson CE (2000) Epidemiology and optimal foraging: modelling the ideal free distribution of insect vectors. Parasitology 120:319–327
Knudsen R, Amundsen PA, Klemetsen A (2002) Parasite-induced host mortality: indirect evidence from a long-term study. Environ Biol Fishes 64:257–265
Krasnov BR, Shenbrot GI, Khokhlova IS, Medvedev SG, Vatschenok VS (1998) Habitat dependence of a parasite–host relationship: flea (Siphonaptera) assemblages in two gerbil species of the Negev desert. J Med Entomol 35:303–313
Krasnov BR, Hastriter MW, Medvedev SG, Shenbrot GI, Khokhlova IS, Vatschenok VS (1999) Additional records of fleas (Siphonaptera) on wild rodents in the Southern part of Israel. Isr J Zool 45:333–340
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NI (2002a) Time of survival under starvation in two flea species (Siphonaptera : Pulicidae) at different air temperatures and relative humidities. J Vector Ecol 27:70–81
Krasnov BR, Khokhlova IS, Oguzoglu I, Burdelova NV (2002b) Host discrimination by two desert fleas using an odour cue. Anim Behav 64:33–40
Krasnov BR, Khokhlova IS, Shenbrot GI (2003a) Density-dependent host selection in ectoparasites: an application of isodar theory to fleas parasitizing rodents. Oecologia 134:365–372
Krasnov BR, Sarfati M, Arakelyan S, Khokhlova IS, Burdelova NV, Degen AA (2003b) Host specificity and foraging efficiency in blood-sucking parasite: feeding patterns of the flea Parapulex chephrenis on two species of desert rodents. Parasitol Res 90:393–399
Krasnov BR, Morand S, Hawlena H, Khokhlova IS, Shenbrot GI (2005) Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 146:209–217
Krasnov BR, Stanko M, Morand S (2006) Age-dependent flea (Siphonaptera) parasitism in rodents: a host’s life history matters. J Parasitol 92:242–248
Laverty TM, Plowright RC (1988) Flower handling by bumblebees—a comparison of specialists and generalists. Anim Behav 36:733–740
Marshall AG (1981) The ecology of ectoparasitic insects. Academic, London
May RM, Anderson RM (1978) Regulation and stability of host-parasite population interactions. 2. Destabilizing processes. J Anim Ecol 47:249–267
McLean JA, Speakman JR (1997) Non-nutritional maternal support in the brown long-eared bat. Anim Behav 54:1193–1204
Menzel R (1985) Learning in honey bees in an ecological and behavioral context. Fortschr Zool 31:55–74
Menzel R, Erber J, Masuhr T (1974) Learning and memory in the honeybee. In: Browne LB (ed) Experimental analysis of insect behaviour. Springer, Berlin
Moore CL (1986) Sex differences in self-grooming of rats: effects of gonadal-hormones and context. Physiol Behav 36:451–455
Murray MD (1987) Effects of host grooming on louse population. Parasitol Today 3:276–278
Neter J, Kutner MH, Nachtsheim CJ, Wasserman W (1996) Applied linear statistical models, 4th edn. McGraw-Hill, Chicago, IL
Papaj DR, Lewis AC (1993) Insect learning. ecological and evolutionary perspectives. Chapman and Hall, London
Pavlov I (1927) Conditioned reflexes. Oxford University Press, Oxford
Poulin R (1993) The disparity between observed and uniform distributions—a new look at parasite aggregation. Int J Parasitol 23:937–944
Poulin R (1996) Sexual inequalities in helminth infections: a cost of being a male? Am Nat 147:287–295
Quinnell RJ (1992) The population-dynamics of Heligmosomoides polygyrus in an enclosure population of wood mice. J Anim Ecol 61:669–679
Samurov MA (1985) Life history and prognosis of abundance of fleas parasitizing jirds of genus Meriones in Volga-Ural sands (in Russian). Ph.D. thesis, Institute of Zoology, Academy of Science of Kazakh SSR, Alma-Ata, USSR
Schalk G, Forbes MR (1997) Male biases in parasitism of mammals: effects of study type, host age, and parasite taxon. Oikos 78:67–74
Shenbrot G (2004) Habitat selection in a seasonally variable environment: test of the isodar theory with the fat sand rat, Psammomys obesus, in the Negev Desert, Israel. Oikos 106:359–365
Sorci G (1996) Patterns of haemogregarine load, aggregation and prevalence as a function of host age in the lizard Lacerta vivipara. J Parasitol 82:676–678
Tinsley RC (2004) Platyhelminth parasite reproduction: some general principles derived from monogeneans. Can J Zool 82:270–291
Turlings TCJ, Wackers FL, Vet LEM, Lewis WJ, Tumlinson JH (1993) Learning of host-finding cues by hymenopterous parasitoids. In: Papaj DR, Lewis AC (eds) Insect learning: ecological and evolutionary perspectives. Chapman and Hall, London, pp 51–78
Vashchenok VS (1988) Fleas—vectors of pathogens causing diseases in humans and animals. Nauka, Leningrad
Walker M, Steiner S, Brinkhof MWG, Richner H (2003) Induced responses of nestling great tits reduce hen flea reproduction. Oikos 102:67–74
Wikel SK (1996) Immunology of the skin. In: Wikel SK (ed) The immunology of host–ectoparasitic arthropod relationships, vol 1. CAB International, Wallingford, UK, pp 1–29
Wilson K, Bjørnstad ON, Dobson AP, Merler S, Poglayen G, Randolph SE, Read AF, Skorping A (2002) Heterogeneities in macroparasite infections: patterns and processes. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP (eds) The ecology of wildlife disease, vol 2. Oxford University Press, Oxford, pp 6–44
Zuk M, McKean KA (1996) Sex differences in parasite infections: patterns and processes. Int J Parasitol 26:1009–1023
Acknowledgments
Tamar Keasar, Judy Stamps, Arnon Tsairi, Michael Friger, Ally Harari, and Burt Kotler were involved in interesting and stimulating discussions. We thank Meital Yogev and Valeria Hochman for technical assistance. The experimental protocol met the requirements of the 1994 Law for the Prevention of Cruelty to Animals (Experiments on Animals) of the State of Israel and was approved by the Ben-Gurion University Committee for the Ethical Care and Use of Animals in Experiments. Financial support during this study was provided by Israel Ministry of Science, Culture and Sport (grant 3-571). This is publication no. 573 of the Mitrani Department of Desert Ecology and no. 233 of the Ramon Science Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hawlena, H., Abramsky, Z. & Krasnov, B.R. Ultimate mechanisms of age-biased flea parasitism. Oecologia 154, 601–609 (2007). https://doi.org/10.1007/s00442-007-0851-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0851-7