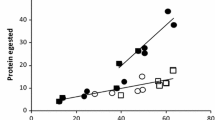
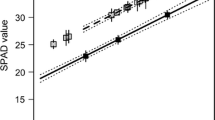
Abstract
Plant carnivory represents an exceptional means to acquire N. Snap traps of Dionaea muscipula serve two functions, and provide both N and photosynthate. Using 13C/15N-labelled insect powder, we performed feeding experiments with Dionaea plants that differed in physiological state and N status (spring vs. autumn plants). We measured the effects of 15N uptake on light-saturated photosynthesis (A max), dark respiration (R D) and growth. Depending on N status, insect capture briefly altered the dynamics of R D/A max, reflecting high energy demand during insect digestion and nutrient uptake, followed by enhanced photosynthesis and growth. Organic N acquired from insect prey was immediately redistributed, in order to support swift renewal of traps and thereby enhance probability of prey capture. Respiratory costs associated with permanent maintenance of the photosynthetic machinery were thereby minimized. Dionaea’s strategy of N utilization is commensurate with the random capture of large prey, occasionally transferring a high load of organic nutrients to the plant. Our results suggest that physiological adaptations to unpredictable resource availability are essential for Dionaea’s success with regards to a carnivorous life style.








Similar content being viewed by others
References
Adamec L (1997) Mineral nutrition of carnivorous plants. Bot Rev 63:273–299
Adamec L (2002) Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytol 155:89–100
Adamec L (2008) The influence of prey capture on photosynthetic rate in two aquatic carnivorous plant species. Aquat Bot 89:66–70
Adamec L (2010) Dark respiration of leaves and traps of terrestrial carnivorous plants: are there greater energetic costs in traps? Cent Eur J Biol 5:121–124
Adamec L (2013) Foliar mineral nutrient uptake in carnivorous plants: what do we know and what should we know? Front Plant Sci 4:1–3
Bennett KF, Ellison AM (2009) Nectar, not colour, may lure insects to their death. Biol Lett 5:469–472
Benzing DH (1987) The origin and rarity of botanical carnivory. Trees 2:364–369
Bergomaz R, Boppré M (1986) A simple instant diet for rearing Arctiidae and other moths. J Lepidopt Soc 40:131–137
Boppré M, Schneider D (1989) The biology of Creatonotos (Lepidoptera: Arctiidae) with special reference to the androconial system. Zool J Linn Soc 96:339–356
Brewer JS, Baker DJ, Nero AS, Patterson AL, Roberts RS, Turner LM (2011) Carnivory in plants as a beneficial trait in wetlands. Aquat Bot 94:62–70
Bruzzese BM, Bowler R, Massicotte HB, Freden AL (2010) Photosynthetic light response in three carnivorous plant species: Drosera rotundifolia, D. capensis and Sarracenia leucophylla. Photosynthetica 48:103–109
Butler JL, Ellison AM (2007) Nitrogen cycling dynamics in the carnivorous northern pitcher plant, Sarracenia purpurea. Funct Ecol 21:835–843
Chase MW, Christenhusz MJM, Sanders D, Fay MF (2009) Murderous plants: Victorian gothic, Darwin and modern insights into vegetable carnivory. Bot J Linn Soc 161:329–356
Darwin C (1875) Insectivorous plants. Murray, London
Ellison AM (2006) Nutrient limitation and stoichiometry of carnivorous plants. Plant Biol 8:740–747
Ellison AM, Adamec L (2011) Ecophysiological traits of terrestrial and aquatic carnivorous plants: are the costs and benefits the same? Oikos 120:1721–1731
Ellison AM, Gotelli NJ (2001) Evolutionary ecology of carnivorous plants. Trends Ecol Evol 16:623–629
Ellison AM, Gotelli NJ (2002) Nitrogen availability alters the expression of carnivory in the northern pitcher plant, Sarracenia purpurea. Proc Natl Acad Sci USA 99:4409–4412
Ellison AM, Gotelli N (2009) Energetics and the evolution of carnivorous plants-Darwin’s ‘most wonderful plants in the world’. J Exp Bot 60:19–42
Escalante-Pérez M, Krol E, Stange A, Geiger D, Al-Rasheid KAS, Hause B, Neher E, Hedrich R (2011) A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proc Natl Acad Sci 108:15492–15497
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:7–19
Farnsworth EJ, Ellison AM (2008) Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. J Ecol 96:213–221
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Field C, Mooney HA (1986) The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of form and function. Cambridge University Press, Cambridge, pp 25–55
Forterre Y, Skotheim JM, Dumals J, Mahadevan L (2005) How the Venus flytrap snaps. Nature 433:421–425
Gibson TC, Waller DM (2009) Evolving Darwin’s ‘most wonderful plant’: ecological steps to a snap-trap. New Phytol 183:575–587
Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD (1984) Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. Am Nat 124:479–497
Greenway W, May J, English S, Whatley FR (1990) Metabolism of [15N]glycine and [13C]glycine by Dionaea muscipula studied by gas chromatography-mass spectrometry. New Phytol 114:581–588
Hajek T, Adamec L (2010) Photosynthesis and dark respiration of leaves of terrestrial carnivorous plants. Biologia 65:69–74
Hanslin HM, Karlsson PS (1996) Nitrogen uptake from prey and substrate as affected by prey capture level and plant reproductive status in four carnivorous plant species. Oecologia 106:370–375
Hutchens JJ, Luken JO (2009) Prey capture in the Venus flytrap: collection or selection? Botany 87:1007–1010
Jaffe MJ (1973) The Role of ATP in mechanically stimulated rapid closure of the Venus’s flytrap. Plant Physiol 51:17–18
Jones FM (1923) The most wonderful plant in the world. Nat Hist 23:589–595
Karagatzides JD, Ellison AM (2009) Construction costs, payback times, and the leaf economics of carnivorous plants. Am J Bot 96:1612–1619
Karlsson PS, Carlsson B (1984) Why does Pinguicula vulgaris L. trap insects? New Phytol 97:25–30
Karlsson PS, Pate JS (1992) Contrasting effects of supplementary feeding of insects or mineral nutrients on the growth and nitrogen and phosphorous economy of pygmy species of Drosera. Oecologia 92:8–13
Knight S (1992) Costs of carnivory in the common bladderwort, Utricularia macrorhiza. Oecologia 89:348–355
Król E, Płachno BJ, Adamec L, Stolarz M, Dziubińska H, Trębacz K (2012) Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann Bot 109:47–64
Kruse J, Adams MA (2008) Sensitivity of respiratory metabolism and efficiency to foliar nitrogen during growth and maintenance. Glob Change Biol 14:1233–1251
Kruse J, Hopmans P, Rennenberg H, Adams MA (2012) Modern tools to tackle traditional concerns: evaluation of site productivity and Pinus radiata management via δ 13C and δ 18O-analysis of tree-rings. For Ecol Manage 285:227–238
Laakkonen L, Jobson RW, Albert VA (2006) A new model for the evolution of carnivory in the bladderwort plant (Utricularia): adaptive changes in cytochrome c oxidase (COX) provide respiratory power. Plant Biol 8:758–764
Lambers H, Chapin FS III, Pons TL (2008) Plant physiological ecology. Springer, New York
Lönnig W-E, Becker H-A (2007) Carnivorous plants. In: Roberts K (ed) Handbook of plant science. Wiley, Milton, pp 1493–1499
Lüttge U (1964) Untersuchungen zur Physiologie der Carnivoren-Drüsen. III. Der Stoffwechsel der resorbierten Substanzen. Flora 155:228–236
Méndez M, Karlsson PS (1999) Costs and benefits of carnivory in plants: insights from the photosynthetic performance of four carnivorous plants in a subarctic environment. Oikos 86:105–112
Moran JA, Moran AJ (1998) Foliar reflectance and vector analysis reveal nutrient stress in prey-deprived pitcher (Nepenthes rafflesiana). Int J Plant Sci 159:996–1001
Pavlovič A, Singerová L, Demko V, Hudák J (2009) Feeding enhances photosynthetic efficiency in the carnivorous pitcher plant Nepenthes talangensis. Ann Bot 104:307–314
Pavlovič A, Demko V, Hudak J (2010) Trap closure and prey retention in Venus flytrap (Dionaea muscipula) temporarily reduces photosynthesis and stimulates respiration. Ann Bot 105:37–44
Pavlovič A, Slováková L, Pandolfi C, Mancuso S (2011) On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). J Exp Bot 62:1991–2000
Rea PA, Whatley FR (1983) The influence of secretion elicitors and external pH on the kinetics of D-alanine uptake by the trap lobes of Dionaea muscipula Ellis. Planta 158:312–319
Rischer H, Hamm A, Bringmann G (2002) Nepenthes insignis uses a C2-portion of the carbon skeleton of L-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemisty 59:603–609
Roberts PR, Oosting HJ (1958) Responses of Venus flytrap (Dionaea muscipula) to factors involved in its endemism. Ecol Monogr 28:193–217
Schulze W, Schulze ED, Schulze I, Oren R (2001) Quantification of insect nitrogen utilization by the Venus flytrap Dionaea muscipula catching prey with highly variable isotope signature. J Exp Bot 52:1041–1049
Schulze W, Sangaard KW, Kreuzer I, Knudsen AD, Bemm F, Thogersen IB, Brautigam A, Thomsen LR, Schliesky S, Dyrlund TF, Escalante-Perez M, Becker D, Schultz J, Karring H, Weber A, Hojrup P, Hedrich R, Enghild JJ (2012) The protein composition of the digestive fluid from the Venus flytrap sheds light on prey digestion mechanisms. Mol Cell Proteomics 11:1306–1319
Small E (1972) Photosynthetic rates in relation to nitrogen recycling as an adaptation to nutrient deficiency in peat bog plants. Can J Bot 50:2227–2233
Stitt M, Hurry V (2002) A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr Opin Plant Biol 5:199–206
Thorén ML, Tuomi J, Kämäräinen T, Laine K (2003) Resource availability affects investment in carnivory in Drosera rotundifolia. New Phytol 159:507–511
Tokunaga T, Takada N, Ueda M (2004) Mechanism of antifeedant activity of plumbagin, a compound concerning the chemical defense in carnivorous plant. Tetrahedron Lett 45:7115–7119
Wakefield AE, Gotelli NJ, Wittmann SE, Ellison AM (2005) Prey addition alters nutrient stoichiometry of the carnivorous plant Sarracenia purpurea. Ecology 86:1737–1743
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Acknowledgments
This research was funded by the European Research Council under the European Union’s Seventh Framework Program (FP/20010-2015)/ERC grant agreement no. (250194-Carnivorom); and by the King Saud University, Riyadh, Saudi Arabia. We thank Monika Eibelmeier and Erika Fischer for excellent technical assistance and Prof. Mark Adams and three anonymous reviewers for many helpful comments on earlier draft(s) of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Colin Mark Orians.
J. Kruse and P. Gao contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kruse, J., Gao, P., Honsel, A. et al. Strategy of nitrogen acquisition and utilization by carnivorous Dionaea muscipula . Oecologia 174, 839–851 (2014). https://doi.org/10.1007/s00442-013-2802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2802-9