Summary
Background
In addition to conventional chemotherapeutic regimens and autologous transplantation, novel agents are now part of the treatment armamentarium against multiple myeloma (MM). To evaluate the presumed benefit of novel agents, we performed an analysis of patients with MM at our institution before and after the availability of novel agents.
Design and methods
In all, 200 consecutive patients with newly diagnosed MM (male = 119; female = 81; median age: 61.5 years) treated at our institution between June 1993 and December 2008 were included in this retrospective analysis. Patient cohorts were defined according to date of diagnosis (before and after 01-Jan-2000, respectively), treatment received (chemotherapy only vs. therapy including novel agents), risk profile (International Staging System (ISS)-stage), and cytogenetic features. Primary focus of the analysis was overall survival (OS).
Results
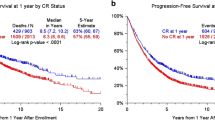
Median OS for MM patients who received conventional chemotherapy was 45.2 months and for patients who received novel agents 74.6 months (P < 0.01). OS for those patients who relapsed after autotransplantation before 2000 was 35.2 months, but 72.7 months (P < 0.01) for those patients with a later relapse. Prolongation of survival for patients receiving novel agents was most evident for patients with ISS stage III (median OS 68.4 vs. 11.2 months for patients with chemotherapy only; P < 0.01). MM patients with an intermediate risk had a longer median OS when receiving novel agents (47.2 vs. 32.8 months).
Conclusion
Treatment with novel agents in MM resulted in a significant prolongation of OS. Benefit of therapy with novel agents was particularly evident for transplant-eligible patients and MM patients with unfavorable prognosis.
Zusammenfassung
Hintergrund
Zusätzlich zur konventionellen Chemotherapie und der autologen Stammzelltransplantation gehören die sog. neuen Substanzen (Thalidomid, Bortezomib, Lenalidomid) nun zur Standardtherapie in der Behandlung des Multiplen Myeloms (MM). Um den Nutzen gegenüber der alleinigen Chemotherapie zu untersuchen, haben wir die Patienten mit Multiplen Myelom an unserer Institution vor und seit der Einführung der neuen Substanzen evaluiert.
Design und Methoden
200 Patienten mit neudiagnostiziertem MM im Zeitraum zwischen Juni 1993 und Dezember 2008 (119 Männer; 81 Frauen; medianes Alter: 61,5 Jahre) wurden in diese retrospektive Analyse eingeschlossen. Patientenkohorten wurden anhand des Zeitpunkts der Erstdiagnose (vor und nach dem 1. Jänner 2000), der Therapie (alleinige Chemotherapie gegenüber Therapie einschließlich der neuen Substanzen), des Risikoprofils (ISS Stadium) und zytogenetischer Faktoren definiert. Primäres Ziel der Analyse war das Gesamtüberleben.
Ergebnisse
Das mediane Gesamtüberleben für Patienten unter konventioneller Chemotherapie betrug 45,2 Monate gegenüber 74,6 Monate bei Behandlung inklusive neuer Substanzen (p < 0,01). Das Gesamtüberleben für Patienten mit Rezidiv nach autologer Transplantation war ebenfalls signifikant unterschiedlich (median 35,2 Monate vs. 72,7 Monate bei Rezidiv vor bzw. nach dem Jahr 2000; p < 0,01). Eine Verlängerung des Gesamtüberlebens unter neuen Substanzen war vor allem für Patienten mit hohem Risikoprofil (ISS Stadium III; 68,4 gegenüber 11,2 Monate; p < 0,01) zu beobachten. Auch Patienten mit einem intermediären Risikoprofil hatten ein längeres Gesamtüberleben mit den neuen Substanzen (47,2 gegenüber 32,8 Monate).
Schlussfolgerung
Behandlung mit neuen Substanzen resultiert in einem verlängerten Gesamtüberleben beim MM. Der Nutzen zeigte sich insbesondere bei Patienten im Rezidiv nach autologer Stammzellentransplantation sowie Patienten mit ungünstigem Risikoprofil.



Similar content being viewed by others
References
UK Myeloma Forum. British Committee for Standards in Haematology. Diagnosis and management of multiple myeloma. Br J Haematol. 2001;115(3):522–40.
Frelay J, Bray F, Sankila R, Parkin DM. EUCAN: cancer incidence, mortality and prevalence in the European Union 1998, version 5.0. IARC Cancer Base No 4. Lyon: IARC Press; 1999.
Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33.
Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. Erratum in J Clin Oncol. 2005;23(25):6281.
Meloma Trialists’ Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1996;16(12):3832–42.
Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma: Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–7.
Child JA, Morgan GJ, Davies FE, et al.: High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N engl J Med. 2003;348(19):1875–83.
Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractoy multiple myeloma. N Engl J med. 1999;341(21):1565–71.
Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–6.
Dimopoulos MA, Spencer A, Attal M, et al. Multiple Myeloma (010) study investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–32. Erratum in N Engl J Med. 2009;361(5):544.
Weber DM, Chen C, Niesvizky R, et al. Multiple Myeloma (009) study investigators. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North america. N Engl J Med. 2007;357(21):2133–42.
Richardson PG, Barlogie B, Berenson J, et al. A phase II study of bortezomib in relapsed, refractory, myeloma. N Engl J Med. 2003;348(26):2609–17.
Richardson PG, Sonneveld P, Schuster MW, et al. Assessment of Proteasome Inhibitor for Extending Remission (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98.
Ludwig H, Avet-Loiseau H, Blade J, et al. European perspective on multiple myeloma treatment strategies: update following recent congresses. Oncologist. 2012;17(5):592–606.
Kumar SK, Rajkumar V, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20.
Steward AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counselling and choice of therapy. Leukemia. 2007;21(3):529–34.
San-Miguel J, Mateos MV, Guiterrez NC. Risk stratification in the era of novel therapies. Cancer J. 2009;15(6):457–64.
Facon T, Mary JY, Hulin C, et al. Intergroup Francophone du Myèlome Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370(9594):1209–18.
Belch A, Reece D, Bahlis NJ. Efficacy and safety of liposomal doxorubicin (DOXIL/CAELYX), bortezomib (Velcade) and dexamethasone in the treatment of previously untreated multiple myeloma patients: impact of cytogenetic profile. Haematologica. 2007;92:180a.
Terpos E, Delimpasi S, Anargyrou K, et al. The combination of bortezomib, doxorubicin, and dexamethasone (PAD) is an effective regimen for high risk, newly diagnosed, patients with multiple myeloma, reduces bone resorption and normalizes angiopoietin-1 to angiopoietin-2 ratio. Blood. 2007;110(11):3596.
Palumbo A, Falco P, Corradini P, et al. GIMEMA-Italian Multiple Myeloma Network. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA-Italian Multiple Myeloma Network. J Clin Oncol. 2007;125(28):4459–65.
Bahlis N, Song K, Trieu Y, et al. Lenalidomide overcomes poor prognosis conferred by del13q and t(4;14) but not del17p13 in multiple myeloma: results of the Canadian MM016 Trial. Blood. 2007;110 Abstract 3597.
Libby E, Ebaid A, Quintana D, Wiggins C. Declining myeloma mortality rates in the united states following introduction of novel therapies. Haematologica. 2011;96(s1):Oral communication-14;32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lamm, W., Eder, S., Bojic, M. et al. Novel agents have a significant impact on survival of patients with multiple myeloma. Wien Klin Wochenschr 127, 92–97 (2015). https://doi.org/10.1007/s00508-014-0605-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-014-0605-6