Abstract
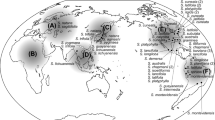
Suaeda subg. Brezia (Chenopodiaceae/Amaranthaceae) comprises ~45 halophytic species distributed worldwide along coastlines and in saline inland habitats. Thirteen species are currently accepted from the Americas, but species delimitation is difficult due to the scarcity of distinguishing characters. Little is known yet about phylogenetic relationships and biogeography of American Brezia species. Here, we present molecular phylogenies based on DNA sequence data from the nuclear ribosomal internal transcribed spacer (ITS) and the chloroplast rpl32-trnL intergenic region. Our sampling comprised 157 accessions covering all 13 American Brezia species along with 38 accessions from 16 Eurasian taxa. Phylogenetic trees were generated using parsimony and Bayesian methods. Three monophyletic lineages were discerned in the ITS tree: the Suaeda maritima, S. prostrata and S. corniculata group. Most American species proved to belong to the S. corniculata group. Species boundaries were mostly not recovered or even contradicted by the ITS data, which could be a consequence of low sequence variation in terminal clades and/or reticulate evolution. The rpl32-trnL phylogeny was poorly resolved, with the majority of American species being part of a polytomy with few supported internal nodes. Several incongruities were found between the nuclear and chloroplast tree, revealing at least four instances of hybridization and chloroplast capture between distant lineages. Chromosome counts showed that all American species are polyploid with hexaploidy prevailing. We discuss our results in terms of species relationships, hybridization, polyploidy and biogeography with emphasis on the colonization from NE Asia and Europe, and the subsequent spread and diversification in the Americas.










Similar content being viewed by others
Notes
1st number—ITS, 2nd number—atpB–rbcL.
Both numbers were also reported for S. calceoliformis by Ferren and Schenk (2003) but tetraploidy is most unlikely in this species because we did not find a respective record in any original publication, and all 23 counts known to us agree in 2n = 54.
References
Alvarado Reyes E, Flores-Olvera H (2013) Suaeda pulvinata (Chenopodiaceae), a new species from saline lakes of central Mexico. Willdenowia 43:300–314
Álvarez I, Wendel JF (2003) Ribosomal ITS sequences and plant phylogenetic inference. Molec Phylogen Evol 29:417–434
Avise JC (1992) Molecular population structure and the biogeographic history of a regional fauna: a case history with lessons for conservation biology. Oikos 63:62–76
Basset IJ, Crompton CW (1978) The genus Suaeda (Chenopodiaceae) in Canada. Canad J Bot 56:581–591
Birdlife International (2014a) East Asia/East Africa Flyway. http://www.birdlife.org/datazone/userfiles/file/sowb/flyways/6_East_Asia_East_Africa_Factsheet. Accessed 20 November 2014
Birdlife International (2014b) Pacific Americas Flyway. http://www.birdlife.org/datazone/userfiles/file/sowb/flyways/1_Pacific_Americas_Factsheet. Accessed 20 November 2014
Blattner FR (1999) Direct amplification of the entire ITS region from poorly preserved plant material using recombinant PCR. Biotechniques 27:1180–1186
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
Cusimano N, Renner S (2014) Ultrametric trees or phylograms for ancestral state reconstruction: does it matter? Taxon 63:721–726
Díaz-Ferguson E, Robinson JD, Silliman B, Wares JP (2010) Comparative phylogeography of North American Atlantic salt marsh communities. Estuaries Coasts 33:828–839
Donoghue MJ (2011) Bipolar biogeography. Proc Natl Acad Sci USA 108:6341–6342
Doyle JJ (1992) Gene trees and species trees: molecular systematics as one-character taxonomy. Syst Bot 17:144–163
Ebrahimzadeh H, Ataei-Azimi A, Akhani H, Noori-Daloi MR (1994) Studies on the caryology of some species of the genus Suaeda (Chenopodiaceae) in Iran. J Sci Iran 5:81–88
Feliner G, Rossello JA (2007) Better the devil you know? Guidelines for insightful utilization of nrDNA in species-level evolutionary studies in plants. Molec Phylogen Evol 44:911–919
Ferren WR, Roberts F (2011) The genus Suaeda (Chenopodiaceae) and conservation of estuaries in the Baja California peninsula and Sonora, Mexico. Proc CNPS Conservation Conference. Sacramento, pp 56–70
Ferren WR, Schenk HJ (2003) Suaeda. In: Flora of North America Edit Committee (ed) Flora of North America 4. Missouri Bot Gard St. Louis, pp 390–398
Ferren WR, Whitmore SA (1983) Suaeda esteroa (Chenopodiaceae), a new species from estuaries of Southern California and Baja California. Madroño 30:181–190
Freitag H, Lomonosova MN (2006) Typification and identity of Suaeda crassifolia, S. prostrata and S. salsa, three often confused species of Suaeda sect. Brezia (Chenopodiaceae, Suaedoideae). Willdenowia 36:21–36
Freitag H, Lomonosova MN (2013) Suaeda. In: Virtual Guide to the Flora of Mongolia. http://www.greif.uni-greifswald.de/floragreif/?cat=13
Hopkins CO, Blackwell WH (1977) Synopsis of Suaeda (Chenopodiaceae) in North America. Sida 7:147–173
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17(8):754–755
Hultén E (1937/1972) Outline of the history of a biota during the Quaternary period: their evolution during and after the glacial period as indicated by the equiformal progressive areas of present plant species Thule, Stockholm, repr Cramer, Lehre
Hultén E (1958) The amphi-atlantic plants and their phytogeographical connections. Kungl Svenska Vetenskapsakademiens Handl Ser 4, vol. 7(1). Almquist & Wiksell, Stockholm
Hultén E (1971) The circumpolar plants II. Dicotyledons. Kungl Svensk Vetenskap Handl Ser 4, vol. 13(1). Almquist & Wiksell, Stockholm
Kadereit JW, Arafeh R, Somogyi G, Westberg E (2005a) Terrestrial growth and marine dispersal? Comparative phylogeography of five coastal plant species at a European scale. Taxon 54:861–876
Kadereit G, Gotzek D, Jacobs S, Freitag H (2005b) Origin and age of Australian Chenopodiaceae. Org Divers Evol 5:59–80
Kadereit G, Ackerly D, Pirie MD (2012) A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proc R Soc London B 279:3304–3311
Kapralov MV, Akhani H, Voznesenskaya EV, Edwards GE, Franceschi VR, Roalson EH (2006) Phylogenetic relationships in the Salicornioideae/Suaedoideae/Salsoloideae s.l. (Chenopodiaceae) clade and a clarification of the phylogenetic position of Bienertia and Alexandra using multiple DNA sequence datasets. Syst Bot 31:571–585
Krahulcová A, Tomšovic P (1997) Ploidy levels in some European representatives of the Suaeda maritima group. Preslia 69:327–332 (in Czech)
Krapp F (2013) Phylogenie und Evolution der Gattung Dyckia (Bromeliaceae). Dissertation. University of Kassel, Germany
Lee JS, Park DS, Ihm BS, Lee WJ (2007) Taxonomic reappraisal on Suaeda australis (Chenopodiaceae) in Korea based on the morphological and molecular characteristics. J Pl Biol 50:605–614
Linder HP, Barker NP (2014) Does polyploidy facilitate long-distance dispersal? Ann Bot (Oxford) 113:1175–1183
Lomonosova MN (2011) Chromosome numbers, taxonomy and distribution of the subgenus Brezia (Suaeda, Chenopodiaceae). Turczaninowia 14:45–52 (in Russian)
Lomonosova M, Freitag H (2003) A new species of Suaeda (Chenopodiaceae) from the Altai. Willdenowia 33:139–147
Lomonosova M, Freitag H (2008) The genus Suaeda (Chenopodiaceae) in Asian Russia. Rastitel´nyj Mir Aziatskoj Rossii 2:12–19 (in Russian)
Lomonosova MN, Freitag H (2009) Chenopodiaceae. In: Marhold K (ed) IAPT/IOPB chromosome data 8, vol. 58. Taxon, pp 1284
Lomonosova MN, Shaulo DN (2010) Karyology of the Siberian representatives of the family Chenopodiaceae. Bot Zhurn (Sankt-Peterbourg) 95:422–426 (in Russian)
Lomonosova MN, Krasnikov AA, Krasnikova SA (2003) Chromosome numbers of the Chenopodiaceae family members of the Kazakhstan flora. Bot Zhurn (Sankt-Peterbourg) 88:134–135 (in Russian)
Lomonosova MN, Krasnikova SA, Krasnikov AA, Sukhorukov AP, Bananova VA, Pavlova NS (2005) Chromosome numbers of Chenopodiaceae species from Russia and Kazakhstan. Bot Zhurn (Sankt-Peterbourg) 90:1132–1134 (in Russian)
Lomonosova MN, Yusupova D, Akopyan JA (2007) Chromosome numbers of the Suaeda (Chenopodiaceae) representatives. Bot Zhurn (Sankt-Peterbourg) 9:1077–1078 (in Russian)
Lomonosova MN, Brandt R, Freitag H (2008) Suaeda corniculata (Chenopodiaceae) and related taxa from Eurasia. Willdenowia 38:81–109
Lorz A (1937) Cytological investigations on five chenopodiaceous genera with special emphasis on chromosome morphology and somatic doubling in Spinacia. Cytologia 8:241–276
Löve A, Löve D (1982) Reports. In: Löve A (ed) IOPB chromosome number reports LXXIV, vol. 31. Taxon, pp 120–126
Maddison WP (1997) Gene trees in species trees. Syst Biol 46:523–536
Milne RI (2006) Northern Hemisphere plant disjunctions: a window on Tertiary land bridges and climate change? Ann Bot (Oxford) 98:465–472
Moore DM (1981) Chromosome numbers of Fuegean angiosperms. Bol Soc Brot Ser 2(53):995–1012
Mulgura ME (1999) Catálogo de las plantas vasculares de la Républica Argentina 2. St. Louis
Müller J, Müller K, Quandt D (2011) PhyDE—Phylogenetic data editor. Version 0.9971. Program distributed by the author
Mulligan GA, Cody WJ (1973) Chenopodiaceae. In: Löve A (ed) IOPB Chromosome number reports XL, vol. 22. Taxon, pp 290
Naciri Y, Linder HP (2015) Species delimitation and relationships: the dance of the seven veils. Taxon 64:3–16
Noguez-Hernández R, Carballo-Carballo A, Flores-Olvera H (2013) Suaeda edulis (Chenopodiaceae), una nueva especie de lagos salinos del centro de México. Bot Sci (Mexico) 91:19–25
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
Pedrol J, Castroviejo S (1988) A proposito del tratamiento taxonomico y nomenclatural del genero Suaeda Forsskål ex Scop. (Chenopodiaceae) en “Flora Iberica”. Anales Jard Bot Madrid 45:93–102
Pirie MD, Humphreys AM, Barker NP, Linder HP (2009) Reticulation, data combination, and inferring evolutionary history: an example from Danthonioideae (Poaceae). Syst Biol 58:612–628
Probatova NC, Rubyka EG, Sokolovskaya AP (1998) Chromosome numbers in vascular plants from islands of Peter the Great Bay and Murayava-Amurskiy Peninsula (Primorsky Krai). Bot Zhurn (Sankt-Peterbourg) 83:125–130 (in Russian)
Scarpino SV, Hunt PJ, Garcia-De-Leon FJ, Juenger TE, Schart M, Kirpatrick M (2013) Evolution of a genetic incompatibility in the genus Xiphophorus. Molec Biol Evol 30:2302–2310
Scarpino SV, Levin DA, Meyers LA (2014) Polyploid formation shapes flowering plant diversity. Amer Naturalist 184:456–465
Schütze P (2011) Molekulare Systematik der Gattung Suaeda (Chenopodiaceae) und Evolution des C4-Photosynthesesyndroms. Dissertation, University of Kassel, Germany
Schütze P, Freitag H, Weising K (2003) An integrated molecular and morphological study of the subfamily Suaedoideae Ulbr. (Chenopodiaceae). Pl Syst Evol 239:257–286
Shaw J, Lickey EB, Schilling EE, Small RL (2007) Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Amer J Bot 95:275–288
Small JK (1933) Manual of the Southeastern Flora. University of N Carolina Press, Chapel Hill
Smirnov YA (1968) Accelerated method for studying somatic chromosomes in fruit trees. Tsitologiya 10:1132–1134 (in Russian)
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0 Beta 10. Sinauer Associations, Sunderland, Massachusetts
Tayalé A, Parisod C (2013) Natural pathways to polyploidy in plants and consequences for genome reorganization. Cytogenet Genome Res 140:79–96
Thiers B (2012) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sciweb.nybg.org/science2/IndexHerbariorum.asp and http://sweetgum.nybg.org/ih/[23.XI.2012]
Watson MC, Ferren WR (1991) A new species of Suaeda (Chenopodiaceae) from coastal Northwestern Sonora, Mexico. Madroño 38:30–36
Weising K, Freitag H (2007) Phylogeography of halophytes from European coastal and inland habitats. Zool Anz 246:279–292
Wen J, Ickert-Bond SM (2009) Evolution of the Madrean Tethyan disjunctions and the North and South American amphitropical disjunctions in plants. J Syst Evol 47:331–348
Yu Y, Harris AJ, He XJ (2010) S-DIVA (Statistical Dispersal-Vicariance Analysis): a tool for inferring biogeographic histories. Molec Phylogen Evol 56:848–850
Yu Y, Harris AJ, Chr Blair, He X (2015) RASP (Reconstruct ancestral state in phylogenies): a tool for historical biogeography. Molec Phylogen Evol 87:46–49
Zakhar’eva OI (1990) Suaeda olufsenii Pauls. In: Takhtajan A (ed) Numeri chromosomatum Magnoliophytorum Florae URSS 1. Aceraceae—Menyanthaceae. Nauka, Leningrad (in Russian)
Acknowledgments
With gratitude, we acknowledge the generous support of a large number of curators for providing herbarium specimens on loan or permitting removal of samples for molecular study in their institutions. This applies in particular to ALA, BKL, CAS, DAO, GR, IEB, LPB, NY, RENO, RSA, SD, TEX, UCSB, and UTC. Other colleagues were helpful by collecting and sending samples taken from herbarium material, as P. W. Ball (Toronto) and C. B. Villamil (Buenos Aires) or fresh material including seeds, as E. Dominguez (Punta Arenas), W. R. Ferren (Santa Barbara), S. Pfanzelt (Mainz), F. G. Schröder (Göttingen), N. Schütz (Stuttgart) and S. Zamudio (Pátzcuaro), E. Nikolin (Yakutsk); and M. Kucev (Barnaul) for providing each two ITS sequences of S. “jacutica” and S. arctica. We are also thankful to W. R. Ferren for joining and guiding our field trips in New Jersey and to J. Schenk (Fullerton), F. Roberts (San Louis Rey) and M. R. Sharifi (Long Beach) in California, as well as for pertinent discussions with the late S. E. Clemants (Brooklyn) and many others. Very kindly W. R. Ferren, E. Dominguez and H. Flores-Olvera also contributed by allocation of images, the latter also by unpublished chromosome counts. We thank the gardeners in Kassel University for their engagement in professionally cultivating American Suaeda plants. We also thank an anonymous reviewer and the editor for useful comments on earlier versions of the manuscript. The project was financially supported by the German Research Foundation (DFG) (Grant WE 1830/7-1 to K. Weising and H. Freitag) and by the Russian Foundation for Basic Research (Grant 12-04-00746 to M. Lomonosova).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Marcus Koch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brandt, R., Lomonosova, M., Weising, K. et al. Phylogeny and biogeography of Suaeda subg. Brezia (Chenopodiaceae/Amaranthaceae) in the Americas. Plant Syst Evol 301, 2351–2375 (2015). https://doi.org/10.1007/s00606-015-1233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-015-1233-y