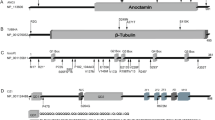
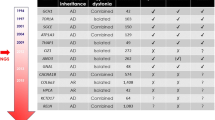
Abstract
Combined and complex dystonias are heterogeneous movement disorders combining dystonia with other motor and/or systemic signs. Although we are beginning to understand the diverse molecular causes of these disease entities, clinical pattern recognition and conventional genetic workup achieve an etiological diagnosis only in a minority of cases. Our goal was to provide a window into the variable genetic origins and distinct clinical patterns of combined/complex dystonia more broadly. Between August 2016 and January 2017, we applied whole-exome sequencing to a cohort of nine patients with varied combined and/or complex dystonic presentations, being on a diagnostic odyssey. Bioinformatics analyses, co-segregation studies, and sequence-interpretation algorithms were employed to detect causative mutations. Comprehensive clinical review was undertaken to define the phenotypic spectra and optimal management strategies. On average, we observed a delay in diagnosis of 23 years before whole-exome analysis enabled determination of each patient’s genetic defect. Whereas mutations in ACTB, ATP1A3, ADCY5, and SGCE were associated with particular phenotypic clues, trait manifestations arising from mutations in PINK1, MRE11A, KMT2B, ATM, and SLC6A1 were different from those previously reported in association with these genes. Apart from improving counseling for our entire cohort, genetic findings had actionable consequences on preventative measures and therapeutic interventions for five patients. Our investigation confirms unique genetic diagnoses, highlights key clinical features and phenotypic expansions, and suggests whole-exome sequencing as a first-tier diagnostic for combined/complex dystonia. These results might stimulate independent teams to extend the scope of agnostic genetic screening to this particular phenotypic group that remains poorly characterized through existing studies.

Similar content being viewed by others
References
Sanger TD, Chen D, Fehlings DL et al (2010) Definition and classification of hyperkinetic movements in childhood. Mov Disord 25:1538–1549
Albanese A, Bhatia K, Bressman SB et al (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord 28:863–873
Marras C, Lang A, van de Warrenburg BP et al (2016) Nomenclature of genetic movement disorders: recommendations of the international Parkinson and movement disorder society task force. Mov Disord 31:436–457
van Egmond ME, Kuiper A, Eggink H et al (2015) Dystonia in children and adolescents: a systematic review and a new diagnostic algorithm. J Neurol Neurosurg Psychiatry 86:774–781
Fung VS, Jinnah HA, Bhatia K, Vidailhet M (2013) Assessment of patients with isolated or combined dystonia: an update on dystonia syndromes. Mov Disord 28:889–898
Lin JP, Lumsden DE, Gimeno H, Kaminska M (2014) The impact and prognosis for dystonia in childhood including dystonic cerebral palsy: a clinical and demographic tertiary cohort study. J Neurol Neurosurg Psychiatry 85:1239–1244
Zech M, Boesch S, Jochim A et al (2017) Clinical exome sequencing in early-onset generalized dystonia and large-scale resequencing follow-up. Mov Disord 32:549–559
van Egmond ME, Lugtenberg CH (2017) Brouwer OF, et al. A post hoc study on gene panel analysis for the diagnosis of dystonia, Mov Disord
Lohmann K, Klein C (2017) Update on the genetics of dystonia. Curr Neurol Neurosci Rep 17:26
Trujillano D, Bertoli-Avella AM, Kumar Kandaswamy K et al (2017) Clinical exome sequencing: results from 2819 samples reflecting 1000 families. Eur J Hum Genet 25:176–182
Jinnah HA, Alterman R, Klein C et al (2017) Deep brain stimulation for dystonia: a novel perspective on the value of genetic testing. J Neural Transm (Vienna) 124:417–430
Lill CM, Mashychev A, Hartmann C et al (2016) Launching the movement disorders society genetic mutation database (MDSGene). Mov Disord 31:607–609
Zech M, Boesch S, Maier EM et al (2016) Haploinsufficiency of KMT2B, encoding the lysine-specific histone methyltransferase 2B, results in early-onset generalized dystonia. Am J Hum Genet 99:1377–1387
Defazio G, Conte A, Gigante AF, Fabbrini G, Berardelli A (2015) Is tremor in dystonia a phenotypic feature of dystonia? Neurology 84:1053–1059
Adzhubei IA, Schmidt S, Peshkin L et al (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249
Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J (2014) A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46:310–315
Landrum MJ, Lee JM, Riley GR et al (2014) ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 42:D980–D985
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Amendola LM, Dorschner MO, Robertson PD et al (2015) Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res 25:305–315
Procaccio V, Salazar G, Ono S et al (2006) A mutation of beta-actin that alters depolymerization dynamics is associated with autosomal dominant developmental malformations, deafness, and dystonia. Am J Hum Genet 78:947–960
Eggink H, van Egmond ME, Verschuuren-Bemelmans CC et al (2017) Dystonia-deafness syndrome caused by a beta-actin gene mutation and response to deep brain stimulation. Mov Disord 32:162–165
Zanotti-Fregonara P, Vidailhet M, Kas A et al (2008) [123I]-FP-CIT and [99mTc]-HMPAO single photon emission computed tomography in a new sporadic case of rapid-onset dystonia-parkinsonism. J Neurol Sci 273:148–151
Anselm IA, Sweadner KJ, Gollamudi S, Ozelius LJ, Darras BT (2009) Rapid-onset dystonia-parkinsonism in a child with a novel atp1a3 gene mutation. Neurology 73:400–401
Tan EK, Refai FS, Siddique M et al (2009) Clinically reported heterozygous mutations in the PINK1 kinase domain exert a gene dosage effect. Hum Mutat 30:1551–1557
Chen DH, Meneret A, Friedman JR et al (2015) ADCY5-related dyskinesia: broader spectrum and genotype-phenotype correlations. Neurology 85:2026–2035
Sim CH, Gabriel K, Mills RD, Culvenor JG, Cheng HC (2012) Analysis of the regulatory and catalytic domains of PTEN-induced kinase-1 (PINK1). Hum Mutat 33:1408–1422
Zimprich A, Grabowski M, Asmus F et al (2001) Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet 29:66–69
Meyer E, Carss KJ, Rankin J et al (2017) Mutations in the histone methyltransferase gene KMT2B cause complex early-onset dystonia. Nat Genet 49:223–237
Savitsky K, Bar-Shira A, Gilad S et al (1995) A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268:1749–1753
Carvill GL, McMahon JM, Schneider A et al (2015) Mutations in the GABA transporter SLC6A1 cause epilepsy with myoclonic-atonic seizures. Am J Hum Genet 96:808–815
Verloes A, Di Donato N, Masliah-Planchon J et al (2015) Baraitser-Winter cerebrofrontofacial syndrome: delineation of the spectrum in 42 cases. Eur J Hum Genet 23:292–301
Miyamoto R, Morino H, Yoshizawa A et al (2014) Exome sequencing reveals a novel MRE11 mutation in a patient with progressive myoclonic ataxia. J Neurol Sci 337:219–223
Havrankova P, Jech R, Roth J, Urgosik D, Ruzicka E (2009) Beneficial effect of deep brain stimulation of GPi in a patient with dystonia-deafness phenotype. Mov Disord 24:465–466
Valente EM, Abou-Sleiman PM, Caputo V et al (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–1160
Stewart GS, Maser RS, Stankovic T et al (1999) The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99:577–587
Directors ABo (2012) Points to consider in the clinical application of genomic sequencing. Genet Med 14:759–761
Need AC, Shashi V, Schoch K, Petrovski S, Goldstein DB (2017) The importance of dynamic re-analysis in diagnostic whole exome sequencing. J Med Genet 54:155–156
Sun Y, Ruivenkamp CA, Hoffer MJ et al (2015) Next-generation diagnostics: gene panel, exome, or whole genome? Hum Mutat 36:648–655
Klein C, Djarmati A, Hedrich K et al (2005) PINK1, Parkin, and DJ-1 mutations in Italian patients with early-onset parkinsonism. Eur J Hum Genet 13:1086–1093
Delia D, Piane M, Buscemi G et al (2004) MRE11 mutations and impaired ATM-dependent responses in an Italian family with ataxia-telangiectasia-like disorder. Hum Mol Genet 13:2155–2163
Fernet M, Gribaa M, Salih MA, Seidahmed MZ, Hall J, Koenig M (2005) Identification and functional consequences of a novel MRE11 mutation affecting 10 Saudi Arabian patients with the ataxia telangiectasia-like disorder. Hum Mol Genet 14:307–318
Carrillo F, Schneider SA, Taylor AM, Srinivasan V, Kapoor R, Bhatia KP (2009) Prominent oromandibular dystonia and pharyngeal telangiectasia in atypical ataxia telangiectasia. Cerebellum 8:22–27
Saunders-Pullman R, Raymond D, Stoessl AJ et al (2012) Variant ataxia-telangiectasia presenting as primary-appearing dystonia in Canadian Mennonites. Neurology 78:649–657
Charlesworth G, Mohire MD, Schneider SA, Stamelou M, Wood NW, Bhatia KP (2013) Ataxia telangiectasia presenting as dopa-responsive cervical dystonia. Neurology 81:1148–1151
Palmer S, Towne MC, Pearl PL et al (2016) SLC6A1 mutation and ketogenic diet in epilepsy with myoclonic-atonic seizures. Pediatr Neurol 64:77–79
Lumsden DE (2017) How might a genetic diagnosis benefit children with dystonia? Eur J Paediatr Neurol 21:245–246
Lohmann E, Gasser T, Grundmann K (2017) Needs and requirements of modern biobanks on the example of dystonia syndromes. Front Neurol 8:9
Acknowledgements
We thank all patients with dystonia and their family members who participated in this study.
Funding
This study was funded by in-house institutional funding from Technische Universität München, Munich, Germany, Helmholtz Zentrum München, Munich, Germany, and Medizinische Universität Innsbruck, Innsbruck, Austria, as well as the Czech Science Foundation (grant: GACR16-13323S), and Charles University, Prague, Czech Republic (project: Progres Q27/LF1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Disclosures concerning the present manuscript
Nothing to report.
Electronic supplementary material
Case 3 (PINK1 mutation) A 56-year-old woman presenting dystonia-parkinsonism syndrome. This video was taken after GPi-DBS at the age 51 years. Dystonic symptoms responded favorably to this intervention, whereas dysarthria and right-sided bradykinesia persisted. Case 5 (MRE11A mutation) A 27-year-old woman presenting generalized dystonia with predominant tremulous cervical dystonia, abnormal upper-limb posturing, and intermittent dystonic trunk movements. Dystonic symptoms were accompanied by myoclonic-like jerky movements. Case 7 (KMT2B mutation) A 13-year-old girl presenting generalized dystonia involving the neck, trunk, and extremities. Dystonia was combined with non-motor features including strabismus, mild facial dysmorphia (bulbous nasal tip), intellectual disability, and hearing impairment. Case 8 (ATM mutation) A 51-year-old woman with segmental cranio-cervical dystonia, consisting of tremulous torticollis and oromandibular dystonia. The latter symptom produced speech impairment. (MOV 27990 kb)
ESM 2
(PDF 102 kb)
Rights and permissions
About this article
Cite this article
Zech, M., Jech, R., Wagner, M. et al. Molecular diversity of combined and complex dystonia: insights from diagnostic exome sequencing. Neurogenetics 18, 195–205 (2017). https://doi.org/10.1007/s10048-017-0521-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-017-0521-9