Abstract
Object
Hepatic lipid accumulation is associated with nonalcoholic fatty liver disease, and the metabolic syndrome constitutes an increasing medical problem. In vivo proton magnetic resonance spectroscopy (1H MRS) allows the assessment of hepatic lipid levels noninvasively and also yields information on the fat composition due to its high spectral resolution.
Materials and methods
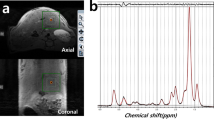
We applied 1H MRS at 9.4T to study lipid content and composition in eight leptin-deficient ob/ob mice as a model of obesity and in four lean ob/+ control mice at 24 weeks of age. PRESS sequence was used. For accurate estimation of signal intensity, differences in relaxation behavior of individual signals were accounted for each mouse individually. Also, in order to minimize spectral degrading due to motion artifacts, respiration gating was applied.
Results
Significant differences between ob/ob and ob/+ control mice were found in both lipid content and composition. The mean chain length was found to be significantly longer in ob/ob mice with a higher fraction of monounsaturated lipids.
Conclusion
1H MRS enables accurate assessment in hepatic lipids in mice, which is attractive for mechanistic studies of altered metabolism given the large number of genetically engineered mouse models available.

Similar content being viewed by others
Abbreviations
- CRLB:
-
The Cramer–Rao lower bound
- fMUL:
-
Fraction of monounsaturated lipids
- FOV:
-
Field-of-view
- fPUL:
-
Fraction of polyunsaturated lipids
- fSL:
-
Fraction of saturated lipids
- fUL:
-
Fraction of unsaturated lipids
- fLM:
-
Fractional lipid mass
- MCL:
-
Mean chain length
- NA:
-
Number of averages
- ob/ob:
-
Lepob/Lepob
- ob/+:
-
Lepob/+
- SL:
-
Saturated lipid component
- TG:
-
Triglyceride
- ULtotal :
-
Total unsaturated lipid component
References
Krssak M, Roden M (2004) The role of lipid accumulation in liver and muscle for insulin resistance and type 2 diabetes mellitus in humans. Rev Endocr Metab Disord 5:127–134
Kovacs P, Stumvoll M (2005) Fatty acids and insulin resistance in muscle and liver. Best Pract Res Clin Endocrinol Metab 19:625–635
Anstee QM, Goldin RD (2006) Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol 87:1–16
Craske JD (1993) Separation of instrumental and chemical errors in the analysis of oils by gas-chromatography—a collaborative evaluation. J Am Oil Chem Soc 70:325–334
Deman JM (1964) Determination of fatty acid composition of milk fat by dual column temperature programmed gas-liquid chromatography. J Dairy Sci 47:546–547
Lindon JC, Holmes E, Nicholson JK (2006) Metabonomics techniques and applications to pharmaceutical research and development. Pharm Res 23:1075–1088
Zancanaro C, Nano R, Marchioro C et al (1994) Magnetic resonance spectroscopy investigations of brown adipose tissue and isolated brown adipocytes. J Lipid Res 35:2191–2199
Knothe G, Kenar JA (2004) Determination of the fatty acid profile by H-1-NMR spectroscopy. Eur J Lipid Sci Tech 106:88–96
Miyake Y, Yokomizo K, Matsuzaki N (1998) Determination of unsaturated fatty acid composition by high-resolution nuclear magnetic resonance spectroscopy. J Am Oil Chem Soc 75:1091–1094
Yeung DKW, Lam SL, Griffith JF et al (2008) Analysis of bone marrow fatty acid composition using high-resolution proton NMR spectroscopy. Chem Phys Lipids 151:103–109
Guillen MD, Ruiz A (2003) H-1 nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. Eur J Lipid Sci Tech 105:502–507
Ruberg FL, Viereck J, Phinikaridou A et al (2006) Identification of cholesteryl esters in human carotid atherosclerosis by ex vivo image-guided proton MRS. J Lipid Res 47:310–317
Ren JM, Dimitrov I, Sherry AD et al (2008) Composition of adipose tissue and marrow fat in humans by H-1 NMR at 7 Tesla. J Lipid Res 49:2055–2062
Hwang JH, Bluml S, Leaf A et al (2003) In vivo characterization of fatty acids in human adipose tissue using natural abundance H-1 clecoupled C-13 MRS at 1.5 T: clinical applications to dietary therapy. NMR Biomed 16:160–167
Lunati E, Farace P, Nicolato E et al (2001) Polyunsaturated fatty acids mapping by (1)H MR-chemical shift imaging. Magn Reson Med 46:879–883
Walling BE, Munasinghe J, Berrigan D et al (2007) Intra-abdominal fat burden discriminated in vivo using proton magnetic resonance spectroscopy. Obesity (Silver Spring) 15:69–77
Lunati E, Marzola P, Nicolato E et al (1999) In vivo quantitative lipidic map of brown adipose tissue by chemical shift imaging at 4.7 tesla. J Lipid Res 40:1395–1400
Strobel K, van den Hoff J, Pietzsch J (2008) Localized proton magnetic resonance spectroscopy of lipids in adipose tissue at high spatial resolution in mice in vivo. J Lipid Res 49:473–480
Lundbom J, Hakkarainen A, Soderlund S et al (2011) Long-TE (1)H MRS suggests that liver fat is more saturated than subcutaneous and visceral fat. NMR Biomed 24:238–245
Bollard ME, Garrod S, Holmes E et al (2000) High-resolution (1)H and (1)H–(13)C magic angle spinning NMR spectroscopy of rat liver. Magn Reson Med 44:201–207
Corbin IR, Furth EE, Pickup S et al (2009) In vivo assessment of hepatic triglycerides in murine non-alcoholic fatty liver disease using magnetic resonance spectroscopy. Bba-Mol Cell Biol L 1791:757–763
Calderan L, Marzola P, Nicolato E et al (2006) In vivo phenotyping of the ob/ob mouse by magnetic resonance imaging and 1H-magnetic resonance spectroscopy. Obesity (Silver Spring) 14:405–414
Griffitts J, Tesiram Y, Reid GE et al (2009) In vivo MRS assessment of altered fatty acyl unsaturation in liver tumor formation of a TGF alpha/c-myc transgenic mouse model. J Lipid Res 50:611–622
Garbow JR, Lin X, Sakata N et al (2004) In vivo MRS measurement of liver lipid levels in mice. J Lipid Res 45:1364–1371
Dimitrov IE, Douglas D, Ren J et al (2011) In vivo determination of human breast fat composition by (1) H magnetic resonance spectroscopy at 7 T. Magn Reson Med 67:20–26
Mosconi E, Fontanella M, Sima DM et al (2011) Investigation of adipose tissues in Zucker rats using in vivo and ex vivo magnetic resonance spectroscopy. J Lipid Res 52:330–336
Lindstrom P (2007) The physiology of obese-hyperglycemic mice [ob/ob mice]. Sci World J 7:666–685
Menahan LA (1983) Age-related changes in lipid and carbohydrate metabolism of the genetically obese mouse. Metabolism 32:172–178
Cheng Y, Zhang J, Shang J et al (2009) Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2 cells by magnesium isoglycyrrhizinate in vitro. Pharmacology 84:183–190
Duncan JG (2008) Lipotoxicity: what is the fate of fatty acids? J Lipid Res 49:1375–1376
Choo HJ, Kim JH, Kwon OB et al (2006) Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49:784–791
Chance DS, Mcintosh MK (1994) Rates of beta-oxidation of fatty-acids of various chain lengths and degrees of unsaturation in highly purified peroxisomes isolated from rat-liver. Comp Biochem Phys B 109:273–280
Shimano H, Matsuzaka T, Yahagi N et al (2007) Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med 13:1193–1202
de Graaf RA, Brown PB, McIntyre S et al (2006) High magnetic field water and metabolite proton T-1 and T-2 relaxation in rat brain in vivo. Magn Reson Med 56:386–394
Delikatny EJ, Chawla S, Leung DJ et al (2011) MR-visible lipids and the tumor microenvironment. NMR Biomed 24:592–611
Giarola M, Rossi B, Mosconi E et al (2011) Fast and minimally invasive determination of the unsaturation index of white fat depots by micro-raman spectroscopy. Lipids 46:659–667
Machann J, Thamer C, Schnoedt B et al (2006) Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized H-1-MR spectroscopy. Magn Reson Med 55:913–917
Johnson NA, Walton DW, Sachinwalla T et al (2008) Noninvasive assessment of hepatic lipid composition: advancing understanding and management of fatty liver disorders. Hepatology 47:1513–1523
Yki-Jarvinen H (2005) Fat in the liver and insulin resistance. Ann Med 37:347–356
Li X, Grundy SM, Patel SB (1997) Obesity in db and ob animals leads to impaired hepatic very low density lipoprotein secretion and differential secretion of apolipoprotein B-48 and B-100. J Lipid Res 38:1277–1288
Dong Z, Dreher W, Leibfritz D et al (2009) Challenges of using MR spectroscopy to detect neural progenitor cells in vivo. Am J Neuroradiol 30:1096–1101
Yahya A, Fallone BG (2010) T(2) determination of the J-coupled methyl protons of lipids: in vivo illustration with tibial bone marrow at 3 T. J Magn Reson Imaging 31:1514–1521
Yahya A, Tessier AG, Fallone BG (2011) Effect of J-coupling on lipid composition determination with localized proton magnetic resonance spectroscopy at 9.4 T. J Magn Reson Imaging 34:1388–1396
Ye Q, Danzer CF, Fuchs A et al (2011) Longitudinal evaluation of intramyocellular lipids (IMCLs) in tibialis anterior muscle of ob/ob and ob/+ control mice using a cryogenic surface coil at 9.4 T. NMR Biomed 24:1295–1301
Acknowledgments
We gratefully acknowledge funding by the Swiss National Science Foundation and SystemsX.ch. We also thank René Tschaggelar and Nikola Cesarovic for excellent technical assistance during various aspects of this study. We especially thank Prof. Peter Boesiger for providing kind access to LCModel and Dr. Anke Henning for advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Q., Danzer, C.F., Fuchs, A. et al. Hepatic lipid composition differs between ob/ob and ob/+ control mice as determined by using in vivo localized proton magnetic resonance spectroscopy. Magn Reson Mater Phy 25, 381–389 (2012). https://doi.org/10.1007/s10334-012-0310-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-012-0310-2