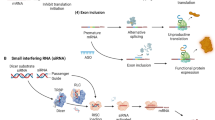
Summary
Oligonucleotide therapeutics are short, single- or double-stranded DNA or RNA molecules consisting of strands of 10–50 nucleotides. By targeted modulation of gene expression oligonucleotides provide the chance of targeting diseases at their molecular level. Within this novel emerging class of compounds oligonucleotide therapeutics are discriminated by their structure, function and mode of action. While antisense oligonucleotides, ribozymes and siRNAs suppress the expression of a protein by complementary hybridizing with their target mRNA, aptamers bind like antibodies to their target protein and thereby inhibit its function. Immunostimulatory oligonucleotides are due to sequence motifs within their nucleotide sequence able to trigger a therapeutic exploitable immune response. Currently, there are only two oligonucleotide therapeutics approved by the FDA, namely the antisense oligonucleotide Fomivirsen and the aptamer Macugen. In this review the mode of action of the diverse oligonucleotide therapeutics and their current status in clinical development will be discussed.
Zusammenfassung
Oligonukleotid Therapeutika sind kurzkettige DNA oder RNA Moleküle, die aus Einzeloder Doppelsträngen von 10–50 Nukleotiden bestehen. Sie ermöglichen gezielt die Expression von Genen zu beeinflussen, um Erkrankungen auf ihrer molekularen Ebene der Entstehung zu therapieren. Innerhalb dieser neuen Substanzklasse unterscheidet man je nach Struktur, Wirkungs- und Funktionsweise zwischen Antisense Oligonukleotiden, Ribozymen, siRNAs, Aptameren und immunstimulatorischen Oligonukleotiden. Während Antisense Oligonukleotide, Ribozyme und siRNAs gezielt die Expression eines Proteins durch komplementäre Basenpaarung an ihre Ziel-mRNA unterdrücken, binden Aptamere ähnlich wie Antikörper spezifisch an ihr Zielprotein und hemmen damit dessen Funktion. Immunstimulatorische Oligonukleotide sind aufgrund bestimmter in ihrer Nukleotidsequenz enthaltener "Sequenzmotive" in der Lage eine therapeutisch nutzbare Immunreaktion hervorzurufen. Derzeit sind mit dem Antisense Oligonukleotid Fomivirsen und dem Aptamer Macugen zwei Oligonukleotide als Arzneimittel zugelassen. Im vorliegenden Artikel sollen die Wirkungsmechanismen der unterschiedlichen Oligonukleotide dargestellt und eine Übersicht über den derzeitigen Entwicklungsstand als Therapeutika in den unterschiedlichen Indikationsbereichen gegeben werden.
Similar content being viewed by others
Literatur
Hopkins AL, Groom CR (2002) The druggable genome. Nat Rev Drug Discov 1: 727–730
Workman P (2004) Drug discovery strategies: technologies to accelerate translation from target to drug. J Chemother 16(Suppl 4): 13–15
Pan WH, Clawson GA (2006) Antisense applications for biological control. J Cell Biochem 98: 14–35
Opalinska JB, Gewirtz AM (2002) Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov 1: 503–514
Pirollo KF, Rait A, Sleer LS, Chang EH (2003) Antisense therapeutics: from theory to clinical practice. Pharmacol Ther 99: 55–77
Stephenson ML, Zamecnik PC (1978) Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A 75: 285–288
Patil SD, Rhodes DG, Burgess DJ (2005) DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J 7: E61–E77
Gleave ME, Monia BP (2005) Antisense therapy for cancer. Nat Rev Cancer 5: 468–479
Kurreck J (2003) Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem 270: 1628–1644
Bayever E, Iversen PL, Bishop MR et al (1993) Systemic administration of a phosphorothioate oligonucleotide with a sequence complementary to p53 for acute myelogenous leukemia and myelodysplastic syndrome: initial results of a phase I trial. Antisense Res Dev 3: 383–390
Vitravene Study Group (2002) A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol 133: 467–474
Jason TL, Koropatnick J, Berg RW (2004) Toxicology of antisense therapeutics. Toxicol Appl Pharmacol 201: 66–83
Ardizzone S, Bianchi PG (2005) Biologic therapy for inflammatory bowel disease. Drugs 65: 2253–2286
Edmondson SR, Thumiger SP, Werther GA, Wraight CJ (2003) Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev 24: 737–764
Wraight CJ, White PJ, McKean SC et al (2000) Reversal of epidermal hyperproliferation in psoriasis by insulinlike growth factor I receptor antisense oligonucleotides. Nat Biotechnol 18: 521–526
Reed JC (2004) Apoptosis mechanisms: implications for cancer drug discovery. Oncology (Williston Park) 18: 11–20
Klasa RJ, Gillum AM, Klem RE, Frankel SR (2002) Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev 12: 193–213
Jansen B, Wacheck V, Heere-Ress E et al (2000) Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet 356: 1728–1733
Marcucci G, Stock W, Dai G et al (2005) Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol 23: 3404–3411
Kirkwood JM, Bedikian AY, Millward MJ et al (2005) Long-term survival results of a randomized multinational phase 3 trial of dacarbazine (DTIC) with or without Bcl-2 antisense (oblimersen sodium) in patients (pts) with advanced malignant melanoma (MM). Proc Am Soc Clin Oncol 24: 7506
Koziner B (2004) Potential therapeutic applications of oblimersen in CLL. Oncology (Williston Park) 18: 32–38
Tamm I (2006) Antisense therapy in malignant diseases: status quo and quo vadis? Clin Sci (Lond) 110: 427–442
Lahn M, Kloeker S, Berry BS (2005) TGF-beta inhibitors for the treatment of cancer. Expert Opin Investig Drugs 14: 629–643
Schlingensiepen KH, Schlingensiepen R, Steinbrecher A et al (2006) Targeted tumor therapy with the TGF-beta2 antisense compound AP 12009. Cytokine Growth Factor Rev 17: 129–139
Chi KN, Eisenhauer E, Fazli L et al (2005) A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2′-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst 97: 1287–1296
Qiu W, Avramoglu RK, Dube N et al (2004) Hepatic PTP-1B expression regulates the assembly and secretion of apolipoprotein B-containing lipoproteins: evidence from protein tyrosine phosphatase-1B overexpression, knockout, and RNAi studies. Diabetes 53: 3057–3066
Olofsson SO, Boren J (2005) Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med 258: 395–410
Rice GP, Hartung HP, Calabresi PA (2005) Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 64: 1336–1342
Myers KJ, Witchell DR, Graham MJ, Koo S, Butler M, Condon TP (2005) Antisense oligonucleotide blockade of alpha 4 integrin prevents and reverses clinical symptoms in murine experimental autoimmune encephalomyelitis. J Neuroimmunol 160: 12–24
Cech TR, Zaug AJ, Grabowski PJ (1981) In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell 27: 487–496
Khan AU (2006) Ribozyme: A clinical tool. Clin Chim Acta 367: 20–27
Rowe PM (1996) Ribozymes enter clinical trials for HIV- 1 treatment. Lancet 348: 1302
McManus MT, Sharp PA (2002) Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 3: 737–747
Cejka D, Losert D, Wacheck V (2006) Short interfering RNA (siRNA): tool or therapeutic? Clin Sci (Lond) 110: 47–58
Soutschek J, Akinc A, Bramlage B et al (2004) Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432: 173–178
Bitko V, Musiyenko A, Shulyayeva O, Barik S (2005) Inhibition of respiratory viruses by nasally administered siRNA. Nat Med 11: 50–55
Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP (2006) Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 5: 123–132
Proske D, Blank M, Buhmann R, Resch A (2005) Aptamers-basic research, drug development, and clinical applications. Appl Microbiol Biotechnol 69: 367–374
Laber DA, Sharma VR, Bhupalam L, Taft B, Hendler FJ, Barnhart KM (2006) Update on the First Phase I Study of AGRO100 in Advanced Cancer. 2005 ASCO Annual Meeting Abstract, 3064 (Abstract)
Klinman DM (2004) Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 4: 249–258
Hornung V, Rothenfusser S, Britsch S et al (2002) Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 168: 4531–4537
Fedulov A, Silverman E, Xiang Y, Leme A, Kobzik L (2005) Immunostimulatory CpG oligonucleotides abrogate allergic susceptibility in a murine model of maternal asthma transmission. J Immunol 175: 4292–4300
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wacheck, V. Oligonukleotid Therapeutika – eine neu entstehende Substanzklasse. Wien Med Wochenschr 156, 481–487 (2006). https://doi.org/10.1007/s10354-006-0331-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10354-006-0331-4