Abstract
Experimental results in rodent medullary slices containing the pre-Bötzinger complex (pre-BötC) have identified multiple bursting mechanisms based on persistent sodium current (I NaP) and intracellular Ca2+. The classic two-timescale approach to the analysis of pre-BötC bursting treats the inactivation of I NaP, the calcium concentration, as well as the Ca2+-dependent inactivation of IP 3 as slow variables and considers other evolving quantities as fast variables. Based on its time course, however, it appears that a novel mixed bursting (MB) solution, observed both in recordings and in model pre-BötC neurons, involves at least three timescales. In this work, we consider a single-compartment model of a pre-BötC inspiratory neuron that can exhibit both I NaP and Ca2+ oscillations and has the ability to produce MB solutions. We use methods of dynamical systems theory, such as phase plane analysis, fast-slow decomposition, and bifurcation analysis, to better understand the mechanisms underlying the MB solution pattern. Rather surprisingly, we discover that a third timescale is not actually required to generate mixed bursting solutions. Through our analysis of timescales, we also elucidate how the pre-BötC neuron model can be tuned to improve the robustness of the MB solution.




















Similar content being viewed by others
References
Afraimovich, V.S., Gonchenko, S.V., Lerman, L.M., Shilnikov, A.L., & Turaev, D.V.(2014). Scientific heritage of L.P. Shilnikov. Part 1. Regular and Chaotic Dynamics, 19(4), 435–460.
Afraimovich, V.S., & Shilnikov, L.P. (1974b). On some global bifurcations connected with the disappearance of a fixed point of saddle-node type. Soviet Mathematics - Doklady, 15, 1761–1765.
Andronov, A.A., & Leontovich, E.A. (1963). Generalization of limit cycles from a separatrix forming a loop and from the separatrix of an equilibrium state of saddle-node type. American Mathematics Society Translations: Series 2, 33, 189–231.
Andronov, A.A., & Vitt, A.A. (1930). Zur theorie des mitmehmens von van der Pol. Archiv für Elektrotechnik XXIV, 99–110.
Best, J., Borisyuk, A., Rubin, J., Terman, D., & Wechselberger, M. (2005). The dynamic range of bursting in a model respiratory pacemaker network. SIAM Journal on Applied Dynamical Systems, 4, 1107–1139.
Beurrier, C., Congar, P., Bioulac, B., & Hammond, C. (1999). Subthalamic nucleus neurosns switch from single-spike activity to burst-firing mode. The Journal of Neuroscience, 19(2), 599–609.
Brocard, F., Shevtsova, N.A., Bouhadfane, M., Tazerart, S., Heinemann, U., Rybak, I.A., & Vinay, L. (2013). Activity-dependent changes in extracellular Ca 2+ and K+ reveal pacemakers in the spinal locomotor-related network. Neuron, 77(6), 1047–1054.
Butera, R.J., Rinzel, J., & Smith, J.C. (1999). Models of respiratory rhythm generation in the pre-B,ötzinger complex. I. Bursting pacemaker neurons. Journal of Neurophysiology, 82(1), 382–397.
Clewley, R., Rotstein, H.G., & Kopell, N. (2005). A computational tool for the reduction of nonlinear ode systems possessing multiple scales. Multiscale Modeling and Simulation, 4, 732–759.
Del Negro, C.A., Hayes, J.A., & Rekling, J.C. (2011). Dendritic calcium activity precedes inspiratory bursts in pre-Bötzinger complex neurons. The Journal of Neuroscience, 31(3), 1017–1022.
Desroches, M., Guckenheimer, J., Krauskopf, B., Kuehn, C., Osinga, H., & Wechselberger, M. (2012). Mixed-mode oscillations with multiple time scales. SIAM Review, 54, 211–288.
Desroches, M., Kaper, T.J., & oscillations, M. Krupa. (2013). Mixed-mode bursting Dynamics created by a slow passage through spike-adding canard explosion in a square-wave burster. Chaos, 23, 046106.
Doedel, E.J. (1981). Auto: a program for the automatic bifurcation analysis of autonomous systems. Congressus Numerantum, 30, 265–284.
Doedel, E.J., Champneys, A.R., Fairgrieve, T.F., Kuznetsov, Y.A., Oldeman, K.E., Paffenroth, R.C., Sanstede, B., Wang, X.J., & Zhang, C. (2009). Auto-07p: continuation and bifurcation software for ordinary differential equations. available from: http://cmvl.cs.concordia.ca/.
Dunmyre, J.R., Del Negro, C.A., & Rubin, J.E. (2011). Interactions of persistent sodium and calcium-activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. Journal of Computational Neuroscience, 31(2), 305–328.
Ermentrout, G.B., & Terman, D.H. (2010). Mathematical foundations of neuroscience. New York: Springer.
Guckenheimer, J., & Holmes, P. (2013). Nonlinear oscillations, dynamical systems, and bifurcations of vector fields. Springer Science & Business Media, 42.
Hodgkin, A.L., & Huxley, A.F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117, 500–544.
Izhikevich, E.M. (2000). Neural excitability, spiking, and bursting. International Journal of Bifurcation and Chaos, 10, 1171–1266.
Jasinski, P.E., Molkov, Y.I., Shevtsova, N.A., Smith, J.C., & Rybak, I.A. (2013). Sodium and calcium mechanisms of rhythmic bursting in excitatory neural networks of the pre-Bötzinger complex: a computational modelling study. The European Journal of Neuroscience, 37, 212–230.
Linaro, D., Champneys, A., Desroches, M., & Storace, M. (2012). Codimension-two homoclinic bifurcations underlying spike adding in the Hindmarsh-Rose burster. SIAM Journal on Applied Dynamical Systems, 11(3), 939–962.
Lukyanov, V., & Shilnikov, L.P. (1978). Some bifurcations of dynamical system with homoclinic structures. Soviet Mathematics - Doklady, 19, 1314–1318.
Mironov, S.L. (2008). Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. Journal of Physiology, 586, 2277–2291.
Morris, C., & Lecar, H. (1981). Voltage oscillations in the barnacle giant muscle fiber. Biophysical Journal, 35(1), 193–213.
Nan, P., Wang, Y., Kirk, V., & Rubin, J.E. (2015). Understanding and distinguishing three-timescale oscillations: case study in a coupled Morris-Lecar system. SIAM Journal on Applied Dynamical Systems, 14, 1518–1557.
Osinga, H.M., Sherman, A., & Tsaneva-Atanasova, K. (2012). Cross-currents between biology and mathematics: The codimension of pseudo-plateau bursting. Discrete and Continuous Dynamical Systems Series A, 32, 2853–2877.
Park, C., & Rubin, J.E. (2013). Cooperation of intrinsic bursting and calcium oscillations underlying activity patterns of model pre-bötzinger complex neurons. Journal of Computational Neuroscience, 34, 345–366.
Rinzel, J. (1987). A formal classification of bursting mechanisms in excitable systems. In Gleason, A.M. editor, Proceedings of the International Congress of Mathematicians, pp. 1578–1594. American Mathematical Society, Providence, RI.
Rinzel, J., & Ermentrout, G.B. (1998). Analysis of neural excitability and oscillations. In Koch, C., & Segev, I. (Eds.) Methods in Neuronal Modeling: From Synapses to Networks, second edition, pp. 251–291. MIT Press, Cambridge, MA.
Rubin, J.E., Hayes, J.A., Mendenhall, J.L., & Del Negro, C.A. (2009). Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proceedings of the National Academy of Sciences of the United States of America, 106, 2939–2944.
Rubin, J.E., & Terman, D. (2002). Geometric singular perturbation analysis of neuronal dynamics. In Fiedler, B. (Ed.) Handbook of Dynamical Systems, (Vol. 2 pp. 93–146). Amsterdam: Elsevier.
Strogatz, S.H. (2014). Nonlinear Dynamics and Chaos: with Applications to Physics, Biology, Chemistry, and Engineering Westview press.
Sheroziya, M.G., von BOhlen, O., Unsicker, K., & Egorov, A.V. (2009). Spontaneous bursting activity in the developing entorhinal cortex. The Journal of Neuroscience, 29, 12131–12144.
Shilnikov, A.L., Calabrese, R.L., & Cymbalyuk, G. (2005). Mechanism of bistability: tonic spiking and bursting in a neuron model. Physics Review E, 71, 056214.
Shilnikov, A.L., & Kolomiets, M.L. (2008). Methods of the qualitative theory for the Hindmarsh-Rose model A case study. International Journal of Bifurcation and Chaos, 18, 1–27.
Shilnikov, L.P. (1963). Some cases of generation of periodic motion from singular trajectories. Mathematics of the USSR - Izvestiya, 61, 443–466.
Shilnikov, L.P., Shilnikov, A., Turaev, D., & Chua, L. (2001). Methods of qualitative theory in nonlinear dynamics. Parts I and II. World Sci.
Tazerart, S., Viemari, J.C., Darbon, P., Vinay, L., & Brocard, F. (2007). Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. Journal of Neurophysiology, 98(2), 613.
Tazerart, S., Vinay, L., & Brocard, F. (2008). The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. The Journal of Neuroscience, 28, 8577–8589.
Terman, D. (1992). The transition from bursting to continuous spiking in excitable membrane models. Journal of Nonlinear Science, 2(2), 135–182.
Toporikova, N., & Butera, R.J. (2011). Two types of independent bursting mechanisms in inspiratory neurons: an integrative model. Journal of Computational Neuroscience, 30(3), 515–528.
Tsuruyama, K., Hsiao, C. -F., & Chandler, S.H. (2013). Participation of a persistent sodium current and calcium-activated nonspecific cationic current to burst generation in trigeminal principal sensory neurons. Journal of Neurophysiology, 110(8), 1903– 1914.
Wang, D., Grillner, S., & Wallen, P. (2006). Effects of flufenamic acid on fictive locomotion, plateau potentials, calcium channels and NMDA receptors in the lamprey spinal cord. Neuropharmocology, 51, 1038–1046.
Zhong, G., Masino, M.A., & Harris-Warrick, R.M. (2007). Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. The Journal of Neuroscience, 27(17), 4507–4518.
Ziskind-Conhaim, L., Wu, L., & Wiesner, E.P. (2008). Persistent sodium current contributes to induced voltage oscillations in locomotor-related hb9 interneurons in the mouse spinal cord. Journal of Neurophysiology, 100 (4), 2254–2264.
Acknowledgments
This work was partially supported by NSF awards DMS 1312508, DMS 1612913.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: David Terman
Appendices
Appendix: A: Adjusting timescales in the dendritic subsystem
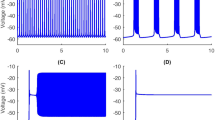
The full model (1a) is capable of generating an MB solution (Fig. 21A) using parameter values for which ([Ca],l) acts as a relaxation oscillator ([IP3]=0.95 μM, A=0.005 μM−1⋅m s −1 as in (Park and Rubin 2013)). However, since the l-nullcline lies extremely close to the left knee of [Ca]-nullcline (Fig. 21B), the trajectory projected to ([Ca],l)-space does not jump up to large [Ca] values and transition to the long burst (LB) phase immediately after passing the left knee (yellow circle). Instead, it slowly moves along the l-nullcline for a transient period before jumping up to the right branch (Fig. 21B and C: from the yellow circle to the green circle); this effect can be interpreted as the influence of the bifurcation in the dendritic subsystem that initiates the (c,l) oscillation, which occurs at a value of [IP3] just below 0.95 (Park and Rubin 2013). Numerically, we observe that small bursts occur during this period (Fig. 21A). Therefore, the existence of the MB solution relies heavily on the nullcline interactions, which can be tuned by changing [IP3]. To clarify the identification of the timescales involved in the MB behavior and the assessment of how to group timescales, and to eliminate the sensitivity of MB solutions to the precise value of [IP3], we increase [IP3] to 1. As a result, the [Ca]-nullcline moves downward and hence further from the l-nullcline. Furthermore, we also slow down l by decreasing A from 0.005 to 0.001 so that l∼O(1000) ms becomes more separated from [Ca], the delay time of the jump up of [Ca] at the left knee is significantly decreased, and the dendritic subsystem takes on a stronger relaxation character.
Time series for attracting solutions of the full model (1a) as well as the bifurcation structure of the dendritic subsystem with parameter values as in Table 1 except [IP3]=0.95[μM], A=0.005[μM−1⋅m s −1] as in (Park and Rubin 2013). Green and yellow symbols mark key points along the solution trajectory (green star: the point with minimum g CAN T o t ; yellow circle: the point when the trajectory passes the left knee of the [Ca]-nullcline; green circle: beginning of the long burst (LB).) (A): Time series for V. (B): Enlarged view of part of Fig. 2B showing the nullclines for [Ca] (red) and l (cyan) for the dendritic subsystem, together with the MB solution from the upper row. (C): Part of time series of [Ca]
Remark 1
Although the dendritic subsystem acts as a more standard relaxation oscillator with the new parameter values ([IP3]=1, A=0.001), [IP3] is not far enough from the bifurcation value for the influence of the bifurcation mentioned above to completely vanish. Hence, there is still a transient delay before [Ca] jumps up at the left knee, but it is much shorter than that with original parameter values.
Appendix: B: Nondimensionalization of the full model (1a)
From numerical simulations, we find that the membrane potential V typically lies between −60 mV and 20 mV. Correspondingly, for x∈{n,h}, we define T x = max(1/τ x (V)) over the range V∈[−60,20] and then define t x (V), a rescaled version of τ x (V), by t x (V)=T x τ x (V). We also define g max to be the maximum of the five conductances g L, g K, g Na, g NaP and g CAN. Furthermore, we let \(G([\text {Ca}])=\frac {[\mathrm {IP_{3}}][\text {Ca}]} {([\mathrm {IP_{3}}]+K_{I})([\text {Ca}]+K_{a})}\) and \(g_{\text {SERCA}}([\text {Ca}])=V_{\text {SERCA}}\frac {[\text {Ca}]}{K^{2}_{\text {SERCA}}+[\text {Ca}]^{2}}\). Substituting these expressions into Eq. (1a) and rearranging, we obtain the following system:
with dimensionless parameters \(\bar {g}_{x}=g_{x}/g_{\max }\) and \(\bar {V}_{x}=V_{x}/Q_{v}\). Note that we have now nondimensionalized the somatic subsystem (1a)-(1c).
Next we deal with Eqs. (1d)-(1e) by nondimensionalizing [Ca], which typically lies between 0 μM and 1 μM, based on numerical simulations. We define G c = max(G 3([Ca])) and G S = max(g SERCA(Ca)) over the range [Ca]∈[0,1] and then define P max to be the maximum of { L IP 3,P IP 3 G c ,G S }. From system (7a), we get the following dimensionless system:
with dimensionless parameters \(\bar {L}_{{\text {IP}}_{3}}=L_{{\text {IP}}_{3}}/P_{\max }\), \(\bar {P}_{{\text {IP}}_{3}}(c)=P_{{\text {IP}}_{3}}/P_{\max }\), \(\bar {g}_{\text {SERCA}}(c)=g_{\text {SERCA}}([\text {Ca}])/P_{\max }\) and \(\bar {K}_{\mathrm {d}}=K_{\mathrm {d}}/Q_{c}\) .
Since we expect V∈[−60,20] and [Ca]∈[0,1], suitable choices for the voltage and calcium scales are Q v =100 mV and Q c =1 μM, respectively. We also see that values of m ∞ (V), m p ∞ (V), f([Ca]), n ∞ (V), h ∞ (V), G([Ca]), \(\bar {g}_{\text {SERCA}}([\text {Ca}])\), n, h and l all lie in the range [0,1]. For the choice of parameters specified in Table 1, the maximum conductance is g Na=28 nS, so we have g max=g Na. Numerical evaluations of 1/τ n (V) and 1/τ h (V) for V∈[−60,20] show that T n ≈20 ms−1 and T h ≈0.04 ms−1. Similarly, we obtain G c ≈0.0421 and G S ≈1000 pL⋅m s −1, so we have P max≈1305 pL⋅m s −1. Using these values we see that all terms in the right hand sides of Eqs. (8a)–(8e) are bounded (in absolute value) by one.
The coefficients of the derivatives in the left hand sides of Eqs. (8a)–(8e) now reveal the relative rates of evolution of the variables. We find that C m /g max=0.75 ms∼O(1) m s, 1/T n =0.05 ms∼O(0.1) ms, 1/T h =25ms∼O(10) m s, \(\frac {\sigma }{P_{\max }\cdot K_{Ca}} = 5.67 \,\text {ms}\sim O(10)\, ms\) and \(\frac {1}{Q_{c}\cdot A} = 200 \,\text {ms} \sim \, O(100)\, ms\). We choose the fast timescale as our reference time, i.e., pick Q t =1 ms, and set
As a result, the dimensionless system (8a) becomes the system (4a) given in Section 3, namely
where R v , R n , R h , R c and R l are dimensionless parameters given in (9a).
Rights and permissions
About this article
Cite this article
Wang, Y., Rubin, J.E. Multiple timescale mixed bursting dynamics in a respiratory neuron model. J Comput Neurosci 41, 245–268 (2016). https://doi.org/10.1007/s10827-016-0616-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-016-0616-6