Abstract
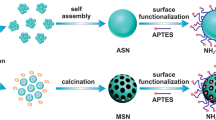
Mesoporous silica material has dual characteristics including adsorption of organic contaminants and transport through the sediments, making it an ideal material as a platform for zerovalent iron particles in the in situ remediation of dense non-aqueous phase liquids such as trichloroethylene. In this paper, tunable adsorption behavior of silica materials was quantitatively investigated by batches of equilibrium experiments. Significant enhancement in adsorption capacity was observed on mesoporous organo-silica particles as a consequence of the functionalization of particle surface from hydrophilicity to hydrophobicity. The fact that there is a wide difference in adsorption capacities between the non-functionalized mesoporous silica (MCM-41) and the alkyl-functionalized mesoporous silica prompted a study to control adsorption levels by simply adjusting the amount of methyl triethoxysilane (MTES) precursor in a mixture of MTES and tetramethoxysilane. In comparison with the most commonly used adsorbent activated carbon, the higher yield of adsorbent of 83 ± 2.6% was observed for mesoporous methyl silica particles. Particle characterizations were performed by means of X-ray powder diffraction, transmission electron microscopy, scanning electron microscopy, Brunauer–Emmett–Teller, thermogravimetric analysis and Fourier transform infrared measurements.








Similar content being viewed by others
References
Hiortdahl KM, Borden RC (2014) Enhanced reductive dechlorination of tetrachloroethene dense nonaqueous phase liquid with EVO and Mg(OH)2. Environ Sci Technol 48:624–631
Mclaren RG, Sudicky EA, Park YJ, Illman WA (2012) Numerical simulation of DNAPL emissions and remediation in a fractured dolomitic aquifer. J Contam Hydrol 136–137:56–71
Jung JG, Do SH, Kwon YJ, Kong SH (2015) Degradation of multi-DNAPLs by a UV/persulphate/ethanol system with the additional injection of a base solution. Environ Technol 36:1044–1049
Dokou Z, Pinder GF (2011) Extension and field application of an integrated DNAPL source identification algorithm that utilizes stochastic modeling and a Kalman filter. J Hydrol 398:277–291
Stroo HF, Leeson A, Marqusee JA, Johnson PC, Ward CH, Kavanaugh MC, Sale TC, Newell CJ, Pennell KD, Lebrón CA, Unger M (2012) Chlorinated ethene source remediation: lessons learned. Environ Sci Technol 46:6438–6447
Baker RS, Nielsen SG, Heron G, Ploug N (2016) How effective is thermal remediation of DNAPL source zones in reducing groundwater concentrations? Ground Water Monit Remediat 36:38–53
Al-abed SR, Chen JL (2001) Transport of trichloroethylene (TCE) in natural soil by electroosmosis. In: Smith JA, Burns SE (eds) Physicochemical groundwater remediation. Springer, New York, pp 91–114
Pérez-De-Mora A, Zila A, Mcmaster ML, Edwards EA (2014) Bioremediation of chlorinated ethenes in fractured bedrock and associated changes in dechlorinating and nondechlorinating microbial populations. Environ Sci Technol 48:5770–5779
Jin B, Rolle M, Li T, Haderlein SB (2014) Diffusive fractionation of BTEX and chlorinated ethenes in aqueous solution: quantification of spatial isotope gradients. Environ Sci Technol 48:6141–6150
Chokejaroenrat C, Comfort S, Sakulthaew C, Dvorak B (2014) Improving the treatment of non-aqueous phase TCE in low permeability zones with permanganate. J Hazard Mater 268:177–184
Lawrinenko M, Wang ZJ, Horton R, Mendivelso-Perez D, Smith EA, Webster TE, Laird DA, Van Leeuwen JH (2017) Macroporous carbon supported zerovalent iron for remediation of trichloroethylene. ACS Sustain Chem Eng 5:1586–1593
Liang SH, Kuo YC, Chen SH, Chen CY, Kao CM (2013) Development of a slow polycolloid-releasing substrate (SPRS) biobarrier to remediate TCE-contaminated aquifers. J Hazard Mater 254–255:107–115
Suárez S, Arconada N, Castro Y, Coronado JM, Portela R, Durán A, Sánchez B (2011) Photocatalytic degradation of TCE in dry and wet air conditions with TiO2 porous thin films. Appl Catal B Environ 108:14–21
Xie W, Yuan S, Mao X, Hu W, Liao P, Tong M, Alshawabkeh AN (2013) Electrocatalytic activity of Pd-loaded Ti/TiO2 nanotubes cathode for TCE reduction in groundwater. Water Res 47:3573–3582
Öztürk Z, Tansel B, Katsenovich Y, Sukop M, Laha S (2012) Highly organic natural media as permeable reactive barriers: TCE partitioning and anaerobic degradation profile in eucalyptus mulch and compost. Chemosphere 89:665–671
Zheng T, Zhan J, He J, Day C, Lu Y, Mcpherson GL, Piringer G, John VT (2008) Reactivity characteristics of nanoscale zerovalent iron-silica composites for trichloroethylene remediation. Environ Sci Technol 42:4494–4499
Zhan J, Zheng T, Piringer G, Day C, Mcpherson GL, Lu Y (2008) Transport characteristics of nanoscale functional zerovalent iron/silica composites for in situ remediation of trichloroethylene. Environ Sci Technol 42:8871–8876
Zheng T, Zhan J, He J, Sunkara B, Lu Y, Mcpherson GL, Piringer G, Kolesnichenko V, John VT (2009) Nanostructured multifunctional materials for environmental remediation of chlorinated hydrocarbons. In: Geiger C, Carvalho-Knighton K (eds) Environmental applications of nanoscale and microscale reactive metal particles. American Chemical Society, Washington, pp 163–179
Shim WG, Lee JW, Moon H (2006) Heterogeneous adsorption characteristics of volatile organic compounds (VOCs) on MCM-48. Sep Sci Technol 41:3693–3719
Parida K, Mishra KG, Dash SK (2012) Adsorption of toxic metal ion Cr(VI) from aqueous state by TiO2-MCM-41: equilibrium and kinetic studies. J Hazard Mater 241–242:395–403
Yang YC, Deka JR, Wu CE, Tsai CH, Saikia D, Kao HM (2017) Cage like ordered carboxylic acid functionalized mesoporous silica with enlarged pores for enzyme adsorption. J Mater Sci 52:6322–6340. doi:10.1007/s10853-017-0864-5
Wang X, Pei Y, Lu M, Lu X, Du X (2015) Highly efficient adsorption of heavy metals from wastewaters by graphene oxide-ordered mesoporous silica materials. J Mater Sci 50:2113–2121. doi:10.1007/s1y0853-014-8773-3
Štandeker S, Novak Z, Knez Ž (2007) Adsorption of toxic organic compounds from water with hydrophobic silica aerogels. J Colloid Interface Sci 310:362–368
Cosnier F, Celzard A, Furdin G, Bégin D, Marêché JF, Barrès O (2005) Hydrophobisation of active carbon surface and effect on the adsorption of water. Carbon 43:2554–2563
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150:468–493
Zhan J, Kolesnichenko I, Sunkara B, He J, Mcpherson GL, Piringer G, John VT (2011) Multifunctional iron-carbon nanocomposites through an aerosol-based process for the in situ remediation of chlorinated hydrocarbons. Environ Sci Technol 45:1949–1954
Zhan J, Sunkara B, Tang J, Wang Y, He J, Mcpherson GL, Piringer G, John VT (2011) Carbothermal synthesis of aerosol-based adsorptive-reactive iron-carbon particles for the remediation of chlorinated hydrocarbons. Ind Eng Chem Res 50:13021–13029
Feng X, Fryxell GE, Wang L-Q, Kim AY, Liu J, Kemner KM (1997) Functionalized monolayers on ordered mesoporous supports. Science 276:923–926
Rao AV, Kulkarni MM (2002) Hydrophobic properties of TMOS/TMES-based silica aerogels. Mater Res Bull 37:1667–1677
Zhao H, Nagy KL, Waples JS, Vance GF (2000) Surfactant-templated mesoporous silicate materials as sorbents for organic pollutants in water. Environ Sci Technol 34:4822–4827
Hu Q, Hampsey JE, Jiang N, Li C, Lu Y (2005) Surfactant-templated organic functionalized mesoporous silica with phosphino ligands. Chem Mater 17:1561–1569
Tanev PT, Pinnavaia TJ (1996) Mesoporous silica molecular sieves prepared by ionic and neutral surfactant templating: a comparison of physical properties. Chem Mater 8:2068–2079
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, Mccullen SB, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114:10834–10843
Araujo AS, Jaroniec M (2000) Thermogravimetric monitoring of the MCM-41 synthesis. Thermochim Acta 363:175–180
Venditti F, Angelico R, Ceglie A, Ambrosone L (2007) Novel surfactant-based adsorbent material for groundwater remediation. Environ Sci Technol 41:6836–6840
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 31671797), Natural Science Foundation of Anhui University (Grant No. 2017KJA123) and the Youth talent support program of Anhui Polytechnic University (Grant No. 2016BJRRC006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ge, F., Ma, Q., Zhu, L. et al. Tunable adsorption behavior of silica materials in the removal of dense non-aqueous phase liquids. J Mater Sci 53, 1801–1809 (2018). https://doi.org/10.1007/s10853-017-1626-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1626-0