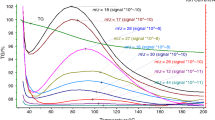
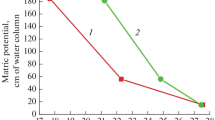
Abstract
Physicochemical aging of soil organic matter is assured by the dynamic character of weak interactions stabilizing its supramolecular structure. However, aging is difficult to monitor, due to low organic matter content in most soils and relatively large time constants. In order to overcome those problems, a model soil, sapric histosol, was exposed to the accelerated aging after a short heating event to 110 °C, and its thermal characteristics were monitored over several months. Classical and temperature modulated differential scanning calorimetry, microcalorimetry and solid-state NMR were used to elucidate the character of involved transitions. The heating event caused separation of an initially broad transition into two processes; a melting, which showed almost no response on the previous heating and a step transition, which is associated with the disruption of water molecule bridges (WaMB) between molecular segments of organic matter. Both processes are preceded by a preparatory phase, starting at subambient temperatures, in which aliphatic domains probably recrystallize and water molecules condensate forming WaMB stabilizing the physical structure of sapric histosol. The aliphatic moieties showed a particular behavior reflected in higher imperfection in crystallite structure upon slow cooling, which was attributed to their interaction with surrounding porous and heterogeneous structures. The results show that soil organic matter aging, considered as a natural process driven by thermodynamic principles, is caused by successive development of WaMB. This is potentially accompanied by recrystallization of aliphatic structures and both processes lead to higher physicochemical stability of soil organic matter.









Similar content being viewed by others
References
Totsche KU, Rennert T, Gerzabek MH, Kögel-Knabner I, Smalla K, Spiteller M, et al. Biogeochemical interfaces in soil: the interdisciplinary challenge for soil science. J Plant Nutr Soil Sci. 2009;173(1):88–99.
Burdon J. Are the traditional concepts of the structures of humic substances realistic? Soil Sci. 2001;166(11):752–69.
Goss K-U, Buschmann J, Schwarzenbach RP. Adsorption of organic vapors to air-dry soils: model predictions and experimental validation. Environ Sci Technol. 2004;38(13):3667–73.
Niederer C, Goss K-U, Schwarzenbach RP. Sorption equilibrium of a wide spectrum of organic vapors in leonardite humic acid: experimental setup and experimental data. Environ Sci Technol. 2006;40(17):5368–73.
Poerschmann J, Kopinke F-D. Sorption of very hydrophobic organic compounds (vhocs) on dissolved humic organic matter (dom). 2. Measurement of sorption and application of a flory–huggins concept to interpret the data. Environ Sci Technol. 2001;35:1142–8.
Kopinke F-D, Poerschmann J, Stottmeister U. Sorption of organic pollutants on anthropogenic humic matter. Environ Sci Technol. 1995;29:941–50.
Xing B, Pignatello JJ. Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter. Environ Sci Technol. 1997;31(3):792–9.
LeBoeuf EJ, Weber WJ. A distributed reactivity model for sorption by soils and sediments. 8. Sorbent organic domains: discovery of a humic acid glass transition and an argument for a polymer-based model. Environ Sci Technol. 1997;31(6):1697–702.
Graber ER, Borisover MD. Hydration-facilitated sorption of specifically interacting organic compounds by model soil organic matter. Environ Sci Technol. 1998;32(2):258–63.
Borisover M, Graber ER. Simplified link solvation model (LSM) for sorption in natural organic matter. Langmuir. 2002;18(12):4775–82.
Kleber M, Johnson MG. Advances in understanding the molecular structure of soil organic matter: implications for interactions in the environment. In: Sparks DL, editor. Advances in agronomy, vol. 106. San Diego: Elsevier Academic Press Inc; 2010. p. 77–142.
Kucerik J, Bursakova P, Prusova A, Grebikova L, Schaumann GE. Hydration of humic and fulvic acids studied by DSC. J Therm Anal Calorim. 2012;110:451–9.
Diehl D. Soil water repellency: dynamics of heterogeneous surfaces. Colloid Surf A. 2013;432:8–18.
Schaumann GE, Bertmer M. Do water molecules bridge soil organic matter molecule segments? Eur J Soil Sci. 2008;59(3):423–9.
Schaumann GE. Matrix relaxation and change of water state during hydration of peat. Colloid Surf A. 2005;265(1–3):163–70.
Hu W-G, Mao J, Xing B, Schmidt-Rohr K. Poly(methylene) crystallites in humic substances detected by nuclear magnetic resonance. Environ Sci Technol. 2000;34:530–4.
Schaumann GE. Soil organic matter beyond molecular structure. 2. Amorphous nature and physical aging. J Plant Nutr Soil Sci. 2006;169(2):157–67.
Piccolo A, Spiteller M. Electrospray ionization mass spectrometry of terrestrial humic substances and their size fractions. Anal Bioanal Chem. 2003;377(6):1047–59.
Piccolo A. The supramolecular structure of humic substances. Soil Sci. 2001;166:810–32.
David J, Weiter M, Vala M, Vynuchal J, Kucerik J. Stability and structural aspects of diketopyrrolopyrrole pigment and its N-alkyl derivatives. Dyes Pigments. 2011;89(2):137–43.
Kucerik J, David J, Weiter M, Vala M, Vynuchal J, Ouzzane I, et al. Stability and physical structure tests of piperidyl and morpholinyl derivatives of diphenyl-diketo-pyrrolopyrroles (DPP). J Therm Anal Calorim. 2012;108(2):467–73.
Schaumann GE. Soil organic matter beyond molecular structure. 1. Macromolecular and supramolecular characteristics. J Plant Nutr Soil Sci. 2006;169(2):145–56.
Stevenson FJ. Humus chemistry: genesis, composition, reactions. New York: Wiley; 1994.
Hatakeyama T, Hatakeyama H. Thermal properties of green polymers and biocomposites, hot topics in thermal analysis and calorimetry. Dordrecht: Kluwer Academic Publishers; 2004.
Wunderlich B. Thermal analysis of polymeric materials. 1st ed. Berlin Heidelberg New York: Springer; 2005.
LeBoeuf EJ. Macromolecular characteristics of natural organic matter and their influence on sorption and desorption behavior of organic chemicals (PhD Dissertation). Michigan, USA: The University of Michigan, 1998.
Chilom G, Rice JA. Glass transition and crystallite melting in natural organic matter. Org Geochem. 2005;36(10):1339–46.
Schaumann GE, LeBoeuf EJ. Glass transitions in peat: their relevance and the impact of water. Environ Sci Technol. 2005;39(3):800–6.
Hurrass J, Schaumann GE. Is glassiness a common characteristic of soil organic matter? Environ Sci Technol. 2005;39(24):9534–40.
Jaeger F. Swelling of soil organic matter and pors size distribution in soil as determined by 1H-NMR-Relaxometry [PhD]. Landau: University Koblenz-Landau; 2011.
Schaumann GE, Thiele-Bruhn S. Molecular modelling of soil organic matter: squaring the circle? Geoderma. 2011;169:55–68.
Schaumann GE, Diehl D, Bertmer M, Jaeger A, Conte P, Alonzo G, et al. Combined proton NMR wideline and NMR relaxometry to study SOM-water interactions of cation-treated soils. J Hydrol Hydromech. 2013;61(1):50–63.
Mikutta R, Schaumann GE, Gildemeister D, Bonneville S, Kramer MG, Chorovere J, et al. Biogeochemistry of mineral-organic associations across a long-term mineralogical soil gradient (0.3–4100 kyr), Hawaiian Islands. Geochim Cosmochim Acta. 2009;73(7):2034–60.
Jäger A, Schaumann GE, Bertmer M. Optimized NMR spectroscopic strategy to characterize water dynamics in soil samples. Org Geochem. 2011;42(8):917–25.
Kunhi Mouvenchery Y, Kučerík J, Diehl D, Schaumann GE. Cation-mediated cross-linking in natural organic matter: a review. Rev Environ Sci Biotechnol. 2012;11(1):41–54.
Schaumann GE, Gildemeister D, Kunhi Mouvenchery Y, Spielvogel S, Diehl D. Interactions between cations and water molecule bridges in soil organic matter. J Soils Sediments. 2013;13(9):1579–88.
Schneckenburger T, Schaumann GE, Woche SK, Thiele-Bruhn S. Short-term evolution of hydration effects on soil organic matter properties and resulting implications for sorption of naphthalene-2-ol. J Soils Sediments. 2012;12(8):1269–79.
Kunhi Mouvenchery Y, Jaeger A, Aquino AJA, Tunega D, Diehl D, Bertmer M, et al. Restructuring of a peat in interaction with multivalent cations: effect of cation type and aging time. PLoS One. 2013;8(6):e65359.
Wunderlich B, Boller A, Okazaki I, Ishikiriyama K. Heat-capacity determination by temperature-modulated DSC and its separation from transition effects. Thermochim Acta. 1997;304(305):125–36.
Seyler RJ, editor. Assignment of the glass transition. Philadelphia: American Society for Testing and Materials; 1994.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109:1203–14.
Dupuy J, Leroy E, Maazouz A. Determination of activation energy and preexponential factor of thermoset reaction kinetics using differential scanning calorimetry in scanning mode: influence of baseline shape on different calculation methods. J Appl Polym Sci. 2000;78(13):2262–71.
Riesen R. Choosing the right baseline. User Com. 2007;25:1–6.
DeLapp RC, Young KD, LeBoeuf EJ. Characterization of thermodynamic properties of several soil- and sediment-derived natural organic materials. Preprints of Extended Abstracts presented at the ACS National Meeting, American Chemical Society, Division of Environmental Chemistry. 2001;41(2):294-8.
Rotaru A, Moanta A, Popa G, Rotaru P, Segal E. Thermal decomposition kinetics of some aromatic azomonoethers. J Therm Anal Calorim. 2009;97(2):485–91.
Rotaru A, Gosa M. Computational thermal and kinetic analysis. J Therm Anal Calorim. 2009;97(2):421–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep. 1971;16:22–31.
Kucerik J, Prusova A, Rotaru A, Flimel K, Janecek J, Conte P. DSC study on hyaluronan drying and hydration. Thermochim Acta. 2011;523(1–2):245–9.
Barany T, Izer A, Menyhard A. Reprocessability and melting behaviour of self-reinforced composites based on PP homo and copolymers. J Therm Anal Calorim. 2010;101(1):255–63.
Menyhard A, Dora G, Horvath Z, Faludi G, Varga J. Kinetics of competitive crystallization of beta- and alpha-modifications in beta-nucleated iPP studied by isothermal stepwise crystallization technique. J Therm Anal Calorim. 2012;108(2):613–20.
Simpson MJ, Simpson AJ. The chemical ecology of soil organic matter molecular constituents. J Chem Ecol. 2012;38(6):768–84.
Sutton R, Sposito G. Molecular structure in soil humic substances: the new view. Environ Sci Technol. 2005;39(23):9009–15.
Feng XJ, Simpson AJ, Simpson MJ. Investigating the role of mineral-bound humic acid in phenanthrene sorption. Environ Sci Technol. 2006;40(10):3260–6.
Simpson MJ, Johnson PCE. Identification of mobile aliphatic sorptive domains in soil humin by solid-state C-13 nuclear magnetic resonance. Environ Toxicol Chem. 2006;25(1):52–7.
Metin S, Hartel RW. Crystallization of fats and oils. In: Shahidi F, editor. Bailey’s industrial oil and fat products. New York: Wiley; 2005. p. 45–76.
Phillips JD. Signatures of divergence and self-organization in soils and weathering profiles. J Geol. 2000;108:91–102.
Kögel-Knabner I, Hatcher PG, Tegelaar EW, De Leeuw JW. Aliphatic components of forest soil organic matter as determined by solid-state carbon-13 NMR and analytical pyrolysis. Sci Total Environ. 1992;113(1–2):89–106.
Marsh KN, editor. Recommended reference materials for the realization of physicochemical properties. Oxford: Blackwell; 1987.
Aquino AJA, Tunega D, Pasalic H, Schaumann GE, Haberhauer G, Gerzabek MH, et al. Molecular dynamics simulations of water molecule-bridges in polar domains of humic acids. Environ Sci Technol. 2011;45(19):8411–9.
Pradzynski CC, Forck RM, Zeuch T, Slavicek P, Buck U. A fully size-resolved perspective on the crystallization of water clusters. Science. 2012;337(6101):1529–32.
Prusova A, Vergeldt FJ, Kucerik J. Influence of water content and drying on the physical structure of native hyaluronan. Carbohydr Polym. 2013;95:515–21.
Simon P, Thomas PS. Some important features of glassines and the nature of amorphous solids. In: Sestak J, Holecek M, Malek J, Kremenakova D, editors. Thermodynamic, structural and behavioral aspects of materials accentuating non-crystalline states. Plzen: O.P.S Nymburk; 2011. p. 248–64.
Steed JW, Atwood JL. Supramolecular chemistry: a concise introduction. 1st ed. Chichester: Wiley; 2000.
Suresh SJ, Naik VM. Hydrogen bond thermodynamic properties of water from dielectric constant data. J Chem Phys. 2000;113(21):9727–32.
Volkov VI, Skirda VD, Vasina EN, Korotchkova SA, Ohya H, Soontarapa K. Self-diffusion of water-ethanol mixture in chitosan membranes obtained by pulsed-field gradient nuclear magnetic resonance technique. J Membr Sci. 1998;138(2):221–5.
Atkins P, De Paula J. Physical chemistry. Oxford: Oxford University Press; 2006.
Siewert C, Demyan M, Kucerik J. Interrelations between soil respiration and its thermal stability. J Therm Anal Calorim. 2012;110:413–9.
Kucerik J, Siewert C, Ctvrtnickova A. Practical application of thermogravimetry in soil science. Part 1: thermal and biological stability of soils from contrasting regions. J Therm Anal Calorim. 2013;113(3):1103–11.
Kucerik J, Siewert C. Practical application of thermogravimetry in soil science Part 2: modeling and prediction of soil respiration using thermal mass losses. J Them Anal Calorim. 2014;116:563–70.
Acknowledgements
Authors would like to thank Dr. Christian Ortmann from TA Instruments, Part of Waters GmbH, Eschborn 65760, Germany for measurement on TAMIII and Yamuna Kunhi Mouvenchery from University of Koblenz-Landau for fruitful discussion. Further the DFG financial support, project SCHA849/8 is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kučerík, J., Schwarz, J., Jäger, A. et al. Character of transitions causing the physicochemical aging of a sapric histosol. J Therm Anal Calorim 118, 1169–1182 (2014). https://doi.org/10.1007/s10973-014-3971-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3971-4