Abstract
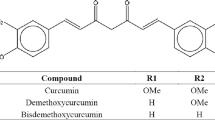
The objective of this study was to enhance the solubility and dissolution rate of a poorly water-soluble antioxidant, curcumin, by fabricating its nanoparticles with two methods: antisolvent precipitation with a syringe pump (APSP) and evaporative precipitation of nanosuspension (EPN). For APSP, process parameters like flow rate, stirring speed, solvent to antisolvent (SAS) ratio, and drug concentration were investigated to obtain the smallest particle size. For EPN, factors like drug concentration and the SAS ratio were examined. The effects of these process parameters on the supersaturation, nucleation, and growth rate were studied and optimized to obtain the smallest particle size of curcumin by both the methods. The average particle size of the original drug was about 10–12 μm and it was decreased to a mean diameter of 330 nm for the APSP method and to 150 nm for the EPN method. Overall, decreasing the drug concentration or increasing the flow rate, stirring rate, and antisolvent amount resulted in smaller particle sizes. Differential scanning calorimetry studies suggested lower crystallinity of curcumin particles fabricated. The solubility and dissolution rates of the prepared curcumin particles were significantly higher than those the original curcumin. The antioxidant activity, studied by the DPPH free radical-scavenging assay, was greater for the curcumin nanoparticles than the original curcumin. This study demonstrated that both the methods can successfully prepare curcumin into submicro to nanoparticles. However, drug particles prepared by EPN were smaller than those by APSP and hence, showed the slightly better solubility, dissolution rate, and antioxidant activity than the latter.





Similar content being viewed by others
References
Aftab N, Vieira A (2010) Antioxidant activities of curcumin and combinations of this curcuminoid with other phytochemicals. Phytother Res 24:500–502
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23:363–398
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818
Cao G, Wang Y (2011) Nanostructures and nanomaterials—synthesis, properties and applications, 2nd edn. World Scientific, London
Dalvi SV, Dave RN (2009) Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind Eng Chem Res 48:7581–7593
Devalapally H, Chakilam A, Amiji MM (2007) Role of nanotechnology in pharmaceutical product development. J Pharm Sci 96:2547–2565
Galindo-Rodriguez S, Allémann SE, Fessi H, Doelker E (2004) Physicochemical parameters associated with nanoparticle formation in the salting salting-out, emulsification–diffusion, and nanoprecipitation methods. Pharm Res 21:1428–1439
Hancock BC, Zografi G (1997) Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci 86:1–12
He Y, Huang Y, Cheng Y (2010) Structure evolution of curcumin nanoprecipitation from a micromixer. Cryst Growth Des 10:1021–1024
Horn D, Rieger J (2001) Organic nanoparticles in the aqueous phase—theory, experiment, and use. Angew Chem Int Ed Engl 40:4330–4361
Jacobs C, Müller RH (2002) Production and characterization of a budesonide nanosuspensions for pulmonary administration. Pharm Res 19:189–194
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14:141–153
Kakran M, Sahoo NG, Li L, Judeh Z, Wang Y, Chong K, Loh L (2010) Fabrication of drug nanoparticles by evaporative precipitation of nanosuspension. Int J Pharm 383:285–292
Kakran M, Sahoo NG, Li L (2011) Dissolution enhancement of quercetin through nanofabrication, complexation, and solid dispersion. Colloids Surf B 88:121–130
Keck CM, Müller RH (2006) Drug nanocrystals of poorly soluble drugs produced by high pressure homogenization. Eur J Pharm Biopharm 62:3–16
Matteucci ME, Hotze MA, Johnston KP, Williams RO 3rd (2006) Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir 22:8951–8959
Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, Leitão SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130
Müller RH, Jacobs C, Kayser O (2000) Nanosuspensions for the formulation of poorly soluble drugs. In: Nielloud F, Marti-Mestres G (eds) Pharmaceutical emulsions and suspensions. Marcel Dekker, New York, pp 383–407
Nielsen AE (1964) Kinetics of precipitation. Pergamon Press, Oxford
Noyes AA, Whitney WR (1897) The rate of solution of solid substances in their own solutions. J Am Chem Soc 19:930–934
Park S-J, Jeon S-Y, Yeo S-D (2006) Recrystallization of a pharmaceutical compound using liquid and supercritical antisolvents. Ind Eng Chem Res 45:2287–2293
Pattekari P, Zheng Z, Zhang X, Levchenko T, Torchilin V, Lvov Y (2011) Top-down and bottom-up approaches in production aqueous nanocolloids of paclitaxel. Phys Chem Chem Phys 13:9014–9019
Rogers TL, Gillespie IB, Hitt JE, Fransen KL, Crowl CA, Tucker CJ, Kupperblatt GB, Becker GN, Wilson DL, Todd C, Broomall CF, Evans JC, Elder EJ (2004) Development and characterization of a scalable controlled precipitation process to enhance the dissolution of poorly water-soluble drugs. Pharm Res 21:2048–2057
Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: the story so far. Eur J Cancer 41:1955–1968
Yen FL, Wu TH, Tzeng CW, Lin LT, Lin CC (2010) Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J Agric Food Chem 58:7376–7382
Zhang HX, Wang JX, Zhang ZB, Le Y, Shen ZG, Chen JF (2009) Micronization of atorvastatin calcium by antisolvent precipitation process. Int J Pharm 374:106–113
Zheng Z, Zhang X, Carbo D, Clark C, Nathan C-A, Lvov Y (2010) Sonication assisted synthesis of polyelectrolyte coated curcumin nanoparticles. Langmuir 26:7679–7681
Acknowledgments
Authors acknowledge Lee Kuan Yew Postdoctoral Fellowship and SUG grant M58050023, NTU, Singapore.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kakran, M., Sahoo, N.G., Tan, IL. et al. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J Nanopart Res 14, 757 (2012). https://doi.org/10.1007/s11051-012-0757-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0757-0