Abstract
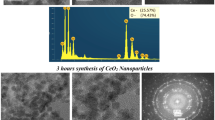
CeO2 nanoparticles with various characteristics find an increasing number of applications in the electronic, medical, and other industries and are therefore likely released in the environment. This calls for investigations linking the physicochemical properties of these particles with their potential environmental impacts. In this study, CeO2 nanoparticle powders were prepared using three different precursors [Ce(NO3)3, CeCl3, and Ce(CH3COO)3] and annealing temperatures (300, 500, and 700 °C). This procedure resulted in nine different types of nanoparticles with differing size (5–90 nm), morphology, surface Ce3+/Ce4+ ratio, and slightly different crystal structures as characterized using transmission electron microscopy, dynamic light scattering, X-ray photoelectron spectroscopy, and X-ray diffraction measurements with Rietveld refinement. These CeO2 nanoparticles underwent toxicity testing at concentrations up to 64 mg L−1 using Daphnia magna. Toxic effects were observed for three particle types with EC50 values between 5 and 64 mg L−1. No clear correlation was observed between the physicochemical properties (size, shape, oxygen occupancy, Ce3+/Ce4+ ratio) of the nanoparticles and their toxicity. However, toxicity was correlated with the amount of Ce remaining suspended in the test medium after 24 h. This indicated that toxic effects may depend on the colloidal stability of CeO2 nanoparticles during the first day of exposure. Therefore, being readily suspended and remaining stable for several days in the aquatic media increases the likelihood that CeO2 nanoparticles will cause unwanted adverse effects.


Similar content being viewed by others
References
Artells E, Issartel J, Auffan M, Borschneck D, Thill A, Tella M et al (2013) Exposure to cerium dioxide nanoparticles differently affect swimming performance and survival in two daphnid species. PLoS ONE 8(8):e71260
ASTM (2014) E729–96. Standard guide for conducting acute toxicity tests on test materials with fishes, macroinvertebrates, and amphibians. doi:10.1520/E0729-96R14
Avgouropoulos G, Ioannides T, Papadopoulou C, Batista J, Hocevar S, Matralis H (2002) A comparative study of Pt/γ-Al2O3, Au/α-Fe2O3 and CuO–CeO2 catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal Today 75(1):157–167
Balzar D, Audebrand N, Daymond M, Fitch A, Hewat A, Langford J et al (2004) Size-strain line-broadening analysis of the ceria round-robin sample. J Appl Crystallogr 37(6):911–924
Bêche E, Charvin P, Perarnau D, Abanades S, Flamant G (2008) Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf Interface Anal 40(3–4):264–267
Bundschuh M, Seitz F, Rosenfeldt RR, Schulz R (2016) Effects of nanoparticles in fresh waters: risks, mechanisms and interactions. Freshw Biol. doi:10.1111/fwb.12701
Cornelis G, Ryan B, McLaughlin MJ, Kirby JK, Beak D, Chittleborough D (2011) Solubility and batch retention of CeO2 nanoparticles in soils. Environ Sci Technol 45(7):2777–2782
Cuahtecontzi-Delint R, Mendez-Rojas MA, Bandala ER, Quiroz MA, Recillas S, Sanchez-Salas JL (2013) Enhanced antibacterial activity of CeO2 nanoparticles by surfactants. Int J Chem React Eng 11(2):1–5
Dabrunz A, Duester L, Prasse C, Seitz F, Rosenfeldt R, Schilde C et al (2011) Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. PLoS One 6(5):e20112
Dahle JT, Livi K, Arai Y (2015) Effects of pH and phosphate on CeO2 nanoparticle dissolution. Chemosphere 119:1365–1371
Deshpande S, Patil S, Kuchibhatla SV, Seal S (2005) Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl Phys Lett 87(13):133113
Fisichella M, Berenguer F, Steinmetz G, Auffan M, Rose J, Prat O (2014) Toxicity evaluation of manufactured CeO2 nanoparticles before and after alteration: combined physicochemical and whole-genome expression analysis in Caco-2 cells. BMC Genom 15(1):1
Gaiser BK, Fernandes TF, Jepson M, Lead JR, Tyler CR, Stone V (2009) Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ Health 8(Suppl 1):S2
Garcia A, Espinosa R, Delgado L, Casals E, González E, Puntes V et al (2011) Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 269(1):136–141
Hunter RJ (2001) Foundations of colloid science. (Oxford University, Ed.), 2nd edn. Oxford University Press, Oxford
Keller AA, Lazareva A (2013) Predicted releases of engineered nanomaterials: from global to regional to local. Environ Sci Technol Lett 1(1):65–70
Kim Y-H, Kim S-K, Kim N, Park J-G, Paik U (2008) Crystalline structure of ceria particles controlled by the oxygen partial pressure and STI CMP performances. Ultramicroscopy 108(10):1292–1296
Korsvik C, Patil S, Seal S, Self WT (2007) Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun 10:1056–1058
Lin W, Huang Y, Zhou X-D, Ma Y (2006) Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol 25(6):451–457
Liu HH, Cohen Y (2014) Multimedia environmental distribution of engineered nanomaterials. Environ Sci Technol 48(6):3281–3292
Liu X, Ray JR, Neil CW, Li Q, Jun Y-S (2015) Enhanced colloidal stability of CeO2 nanoparticles by ferrous ions: adsorption, redox reaction, and surface precipitation. Environ Sci Technol 49(9):5476–5483
López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2010) XAS corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem 58(6):3689
Manier N, Bado-Nilles A, Delalain P, Aguerre-Chariol O, Pandard P (2013) Ecotoxicity of non-aged and aged CeO2 nanomaterials towards freshwater microalgae. Environ Pollut 180:63–70
Patil S, Sandberg A, Heckert E, Self W, Seal S (2007) Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 28(31):4600–4607
Quik JTK, Lynch I, Van Hoecke K, Miermans CJH, Schamphelaere KACD, Janssen CR et al (2010) Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water. Chemosphere 81(6):711–715
Seitz F, Bundschuh M, Rosenfeldt RR, Schulz R (2013) Nanoparticle toxicity in Daphnia magna reproduction studies: the importance of test design. Aquat Toxicol 126:163–168
Seitz F, Rosenfeldt R, Müller M, Lüderwald S, Schulz R, Bundschuh M (2016) Quantity and quality of natural organic matter influence the ecotoxicity of titanium dioxide nanoparticles. Nanotoxicology 1. doi:10.1080/17435390.2016.1222458
Tarnuzzer RW, Colon J, Patil S, Seal S (2005) Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett 5(12):2573–2577
Thompson P, Cox D, Hastings J (1987) Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J Appl Crystallogr 20(2):79–83
Van Hoecke K, Quik JT, Mankiewicz-Boczek J, Schamphelaere KAD, Elsaesser A, der Meeren PV et al (2009) Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ Sci Technol 43(12):4537–4546
Van Hoecke K, De Schamphelaere KA, Van der Meeren P, Smagghe G, Janssen CR (2011) Aggregation and ecotoxicity of CeO2 nanoparticles in synthetic and natural waters with variable pH, organic matter concentration and ionic strength. Environ Pollut 159(4):970–976
Zhang F, Chan S-W, Spanier JE, Apak E, Jin Q, Robinson RD, Herman IP (2002) Cerium oxide nanoparticles: size-selective formation and structure analysis. Appl Phys Lett 80(1):127–129
Acknowledgments
This work was supported by a fellowship in the frame of the research initiative of the Ministry of Education, Science, Further Education and Culture, Rheinland-Pfalz, Germany, in the framework of the research project AufLand. The authors would like to thank James Dillon (SUNY Polytechnic Institute) for his assistance with the background materials on this paper and Dr. Michael Hatzistergos (SUNY Polytechnic Institute) for his guidance and assistance with the XPS data reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alam, B., Philippe, A., Rosenfeldt, R.R. et al. Synthesis, characterization, and ecotoxicity of CeO2 nanoparticles with differing properties. J Nanopart Res 18, 303 (2016). https://doi.org/10.1007/s11051-016-3613-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3613-9