Abstract
Background and aims
Forest ecosystems may act as sinks for or source of atmospheric CO2. While inorganic nitrogen (N) fertilization increases aboveground tree biomass, the effects on soil and rhizosphere microorganisms are less clear, indicating potentially unpredictable changes in nutrient cycling processes maintaining ecosystem functioning. Although plant-derived carbon (C) is the main C source in soils during the vegetation period, information on the response of rhizosphere bacteria assimilating rhizodeposits to increased soil N availability mainly for trees is missing.
Methods
We performed a greenhouse experiment with 13C-CO2 labelled young beech seedlings grown under different N fertilization levels. DNA Stable Isotope Probing (DNA-SIP) in combination with TRFLP and pyrosequencing enabled us to identify bacteria assimilating plant-derived C and to assess the main responders phylogenetically.
Results
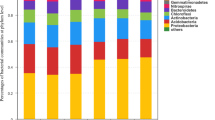
Although above- and belowground allocation of recently fixed photosynthates remained unchanged, microbial rhizosphere community composition was clearly affected by fertilization. Besides, we found lower 13C incorporation into microbial biomass in fertilized soil. Moreover, it could be shown that only a small subset of the rhizosphere microbiome incorporated recently fixed C into its DNA, dominated by Proteobacteria (Alpha- and Betaproteobacteria) and Actinobacteria (Actinomycetales).
Conclusions
Our results suggest that N fertilization may change both the diversity of bacterial communities using rhizodeposits and assimilation rates of recently fixed photosynthates. Given the close interaction of beneficial and/or deleterious microbes and plants in the rhizosphere, this could potentially have positive or negative implications for plant performance on long-term.



Similar content being viewed by others
References
Ai C, Liang G, Sun J, Wang X, He P, Zhou W, He X (2015) Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol Biochem 80:70–78
Bach HJ, Tomanova J, Schloter M, Munch JC (2002) Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. J Microbiol Methods 49:235–245
Ball AS, Drake BG (1997) Short-term decomposition of litter produced by plants grown in ambient and elevated atmospheric CO2 concentrations. Glob Chang Biol 3:29–35
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. Forest Ecol Manag 196:43–56
Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95
Donn S, Kirkegaard JA, Perera G, Richardson AE, Watt M (2015) Evolution of bacterial communities in the wheat crop rhizosphere. Environ Microbiol 17:610–621
Erisman JW, de Vries W (2000) Nitrogen deposition and effects on European forests. Environ Rev 8:65–93
Fierer N, Schimel JP, Holden PA (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Fisk MC, Fahey TJ (2001) Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hardwood forests. Biogeochem. 53:201–223
Forge TA, Simard SW (2001) Short-term effects of nitrogen and phosphorus fertilizers on nitrogen mineralization and trophic structure of the soil ecosystem in forest clearcuts in the southern interior of British Columbia. Can J Soil Sci 81:11–20
Giardina CP, Binkley D, Ryan MG, Fownes JH, Senock RS (2004) Belowground carbon cycling in a humid tropical forest decreases with fertilization. Oecologia 139:545–550
Gschwendtner S, Esperschütz J, Buegger F, Reichmann M, Müller M, Munch JC, Schloter M (2011) Effects of genetically modified starch metabolism in potato plants on photosynthate fluxes into the rhizosphere and on microbial degraders of root exudates. FEMS Microbiol Ecol 76:564–575
Gschwendtner S, Leberecht M, Engel M, Kublik S, Dannenmann M, Polle A, Schloter M (2015) Effects of elevated atmospheric CO2 on microbial community structure at the plant-soil interface of young beech trees (Fagus sylvatica L.) grown at two sites with contrasting climatic conditions. Microb Ecol 69:867–878
Hernández M, Dumont MG, Yuan Q, Conrad R (2015) Different bacterial populations associated with the roots and rhizosphere of rice incorporate plant-derived carbon. Appl. Environ, Microbiol
Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, Johnson DW, Minkkinen K, Byrne KA (2007) How strongly can forest management influence soil carbon sequestration? Geoderma 137:253–268
Joergensen RG (1995) The fumigation-extraction method to estimate soil microbial biomass: extraction with 0.01 M CaCl2. Agribiol Res 48:319–324
Kalyuzhnaya MG, Lapidus A, Ivanova N, Copeland AC, McHardy AC, Szeto E, Salamov A, Grigoriev IV, Suciu D, Levine SR, Markowitz VM, Rigoutsos I, Tringe SG, Bruce DC, Richardson PM, Lidstrom ME, Chistoserdova L (2008) High-resolution metagenomics targets specific functional types in complex microbial communities. Nat Biotechnol 26:1029–1034
King JS, Pregitzer KS, Zak DR, Holmes WE, Schmidt K (2005) Fine root chemistry and decomposition in model communities of north-temperate tree species show little response to elevated atmospheric CO2 and varying soil resource availability. Oecologia 146:318–328
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci Soc Am J 48:1267–1272
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar BA, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucl Acids Res 32:1363–1371
Lueders T, Manefield M, Friedrich MW (2004a) Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ Microbiol 6:73–78
Lueders T, Wagner B, Claus P, Friedrich MW (2004b) Stable isotope probing of rRNA and DNA reveals a dynamic methylotroph community and trophic interactions with fungi and protozoa in oxic rice field soil. Environ Microbiol 6:60–72
Majdi H, Andersson P (2005) Fine root production and turnover in a Norway spruce stand in Northern Sweden: effects of nitrogen and water manipulation. Ecosystems 8:191–199
Majdi H, Kangas P (1997) Demography of fine roots in response to nutrient applications in a Norway spruce stand in Southwestern Sweden. Ecoscience 4:199–205
Majdi H, Ohrvik J (2004) Interactive effects of soil warming and fertilization on root production, mortality, and longevity in a Norway spruce stand in Northern Sweden. Glob Chang Biol 10:182–188
Mao Y, Li X, Smyth EM, Yannarell AC, Mackie RI (2014) Enrichment of specific bacterial and eukaryotic microbes in the rhizosphere of switchgrass (Panicum virgatum L.) through root exudates. Environ Microbiol Rep 6:293–306
Marx M, Buegger F, Gattinger A, Zsolnay Á, Munch JC (2007) Determination of the fate of 13C labelled maize and wheat exudates in an agricultural soil during a short-term incubation. Eur J Soil Sci 58:1175–1185
Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014) Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8:1577–1587
Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, Buckler ES, Ley RE (2013) Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci U S A 110:6548–6553
Percival DC, Proctor JTA, Sullivan JA (2001) Cultivar differences in carbon assimilation and partitioning of primocane-fruiting raspberry. J Am Pomol Soc 55:82–89
Phillips RP, Fahey TJ (2007) Fertilization effects on fineroot biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Phytol. 176:655–664
Pilloni G, Granitsiotis MS, Engel M, Lueders T (2012) Testing the limits of 454 pyrotag sequencing: reproducibility, quantitative assessment and comparison to T-RFLP fingerprinting of aquifer microbes. PLoS One 7:e40467
Prescott CE, Corbin JP, Parkinson D (1992) Immobilization and availability of N and P in the forest floors of fertilized Rocky Mountain coniferous forests. Plant Soil 143:1–10
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moenne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18:1918–1927
Sauer D, Kuzyakov Y, Stahr K (2006) Spatial distribution of root exudates of five plant species as assessed by C14 labeling. J Plant Nutr Soil Sci 169:360–362
Schloss PD (2009) A high-throughput DNA sequence aligner for microbial ecology studies. PLoS One 4(12):e8230
Stinson G, Kurz WA, Smyth CE, Neilson ET, Dymond CC, Metsaranta JM, Boisvenue C, Rampley GJ, Li Q, White TM, Blain D (2011) An inventory-based analysis of Canada's managed forest carbon dynamics, 1990 to 2008. Glob Chang Biol 17:2227–2244
Tamm CO (1991) Nitrogen in terrestrial ecosystems: questions of productivity, Vegetational changes, and ecosystem stability. Springer, Berlin, Germany
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Vance ED, Brooks PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wallenstein MD, Peterjohn WT, Schlesinger WH (2006) N fertilization effects on denitrification and N cycling in an aggrading forest. Ecol Appl 16:2168–2176
Werner RA, Brand WA (2001) Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun Mass Spectrom 15:501–519
Wertz S, Leigh AKK, Grayston SJ (2012) Effects of long-term fertilization of forest soils on potential nitrification and on the abundance and community structure of ammonia oxidizers and nitrite oxidizers. FEMS Microbiol Ecol 79:142–154
Yanai RD, Majdi H, Park BB (2003) Measured and modelled differences in nutrient concentrations between rhizosphere and bulk soil in a Norway spruce stand. Plant Soil 257:133–142
Yue JC, Clayton MK (2005) A similarity measure based on species proportions. Commun Stat 34:2123–2131
Zhu B, Panke-Buisse K, Kao-Kniffin J (2015) Nitrogen fertilization has minimal influence on rhizosphere effects of smooth crabgrass (Digitaria ischaemum) and bermudagrass (Cynodon dactylon). J Plant Ecol 8:390–400
Acknowledgments
We gratefully acknowledge Susanne Kublik and Kornelia Galonska for their assistance in molecular analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Liesje Mommer.
Electronic supplementary material
ESM 1
Abundance of 16S rRNA genes ng-1 DNA among 10 gradient fractions of DNA extracted from (a) unfertilized and (b) fertilized soils after 2, 4, 7 and 10 days of growth in 13C-CO2 labelled and unlabeled atmosphere quantified via real-time PCR (n = 1). (GIF 48 kb)
ESM 2
(PDF 346 kb)
ESM 3
UPGMA dendrogram generated from TRFLP profiles based on 16S rRNA gene amplicons from 12 gradient fractions (numbered 1–12) of DNA extracted from (a) unfertilized (4 days) and (b) fertilized (7 days) soils obtained from unlabeled (C12) and labelled (C13) pots (n = 3). Buoyant density of the gradient fractions increased with decreasing fraction number. The scale indicates the distance level. (GIF 52 kb)
ESM 4
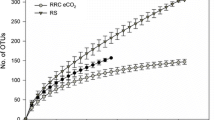
Rarefaction curves of partial 16S rRNA gene sequences after DNA extraction and PCR amplification obtained from heavy (h) and medium (m) fractions from unfertilized (f0, 4 days) and fertilized (f10, 7 days) soils and unlabeled (C12) and labelled (C13) pots at 97 % similarity level normalized with respect to sample size (n = 1). (GIF 66 kb)
ESM 5
(PDF 125 kb)
ESM 6
Phylogenetic dendrogram (maximum likelihood consensus tree) showing the distribution of sequences related to (a) Proteobacteria and (b) Actinobacteria derived from unfertilized and fertilized rhizosphere soil after 4 and 7 days of incubation, respectively. OTUs representing bacteria assimilating plant-derived C are highlighted in grey. (GIF 132 kb)
Rights and permissions
About this article
Cite this article
Gschwendtner, S., Engel, M., Lueders, T. et al. Nitrogen fertilization affects bacteria utilizing plant-derived carbon in the rhizosphere of beech seedlings. Plant Soil 407, 203–215 (2016). https://doi.org/10.1007/s11104-016-2888-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2888-z