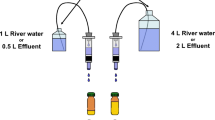
Abstract
Background, aim, and scope
The enzyme-linked receptor assay (ELRA) detects estrogenic and anti-estrogenic effects at the molecular level of receptor binding and is a useful tool for the integrative assessment of ecotoxicological potentials caused by hormonally active agents (HAA) and endocrine disrupting compounds (EDC). The main advantage of the ELRA is its high sample throughput and its robustness against cytotoxicity and microbial contamination. After a methodological adaptation to salinity of the ELRA, according to the first part of this study, which increased its salinity tolerance and sensitivity for 17-β-estradiol, the optimised ELRA was used to investigate 13 native sediments characterised by different levels of salinity and chemical contamination. The applicability of the ELRA for routine analysis in environmental assessment was evaluated. Salinity is often a critical factor for bioassays in ecotoxicological sediment assessment. Therefore, salinity of the samples was additionally adjusted to different levels to characterise its influence on elution and binding processes of receptor-binding substances.
Materials and methods
The ELRA was carried out with the human estrogen receptor α (ER) in a 96-well microplate format using the experimental setup known from the competitive immunoassay based on ligand–protein interaction. It is an important improvement that a physiologically relevant receptor was used as a linking protein instead of an antibody. The microplates were coated with a 17-β-estradiol-BSA conjugate, and dilution series of estradiol and of native sediment samples were added and incubated with the ER. After a washing step, a biotinylated mouse anti-ER antibody was added to each well. Receptor binding to estradiol, agonistic and antagonistic receptor binding, were determined by a streptavidin-POD-biotin complex with subsequent measurement of the peroxidase activity at the wavelength of 450 nm using a commercial ELISA multiplate reader. The sediment elutriates and pore water samples of sediments were tested in a dilution series to evaluate at which dilution step the receptor-binding potential ends. In the elution process (see Section 2.1 to 2.2), a method was developed to adjust the salinity to the levels of the reference testings, which offers an appropriate option to adjust the salinity in both directions. Statistical evaluation was made with a combination of the Mann–Whitney U test and the pT-method.
Results
This part of the study characterised the environmental factor ‘salinity’ for prospective applications of the ELRA. Using reference substances such as 17-β-estradiol, the ELRA showed sigmoid concentration-effect relations over a broad range from 0.05 μg/l to 100 μg/l under physiological conditions. After methodological optimisation, both sensitivity and tolerance of the assay against salinity could be significantly raised, and the ELRA became applicable under salinity conditions up to concentrations of 20.5‰. The mean relative inter-test error (n = 3) was around 11% with reference substances and below 5% for single sediments elutriates in three replicates each. For sediment testings, the pore water and different salinity-adjusted elutriates of 13 sediments were used. A clear differentiation of the receptor-binding potential could be reached by application of the pT-method. Thereby, pT-values from one to six could be assigned to the sediments, and the deviation caused by the different salinity conditions was one pT-value. The mean standard deviation in the salinity adaptation procedure of the elutriates was below 5%.
Discussion
Although the ELRA has already been used for assessments of wastewater, sludge and soil, its applicability for samples to different salinity levels has not been investigated so far. Even if the ELRA is not as sensitive as the E-screen or the YES-assay, with regard to reference substances like 17-β-estradiol, it is a very useful tool for pre-screening, because it is able to integrate both estrogenic as well as anti-estrogenic receptor-binding effects. According to the results of sediment testing, and given the integrative power to detect different directions of effects, the ELRA shows sufficient sensitivity and salinity tolerance to discriminate receptor-binding potentials in environmental samples.
Conclusions
The optimised ELRA assay is a fast, cost-effective, reliable and highly reproducible tool that can be used for high-throughput screening in a microplate format in detecting both estrogenic and anti-estrogenic effects. Additionally, the ELRA is robust against microbial contaminations, and is not susceptible towards cytotoxic interferences like the common cell-culture methods. The general applicability and sufficient sensitivity of the ELRA was shown in freshwater environments. Marine and brackish samples can be measured up to salinity levels of 20.5‰.
Recommendations and perspectives
In view of the proven sensitivity, functionality and the fastness of the ELRA, it is recommendable to standardise the test method. At the moment, no adequate in vitro test procedure exists which is standardised to DIN or ISO levels. The E-screen and the yeast estrogen/androgen screens (YES/YAS) sometimes underlie strong cytotoxic effects, as reported in the first part of this study. Further development of an ELRA assay using human androgen receptors appears to be very promising to gain information about androgenic and anti-androgenic effects, too. This would offer a possibility to use the ELRA as a fast and reliable pre-screening tool for the detection of endocrine potentials, thus minimising time and cost-expensive animal experiments.









Similar content being viewed by others
References
Ankley G, Mihaich E, Stahl R, Tillitt D, Colborn T, McMaster S, Miller R, Bantle J, Campbell P, Denslow N, Dickerson R, Folmar L, Fry M, Giesy J, Gray E, Guiney P, Hutchinson T, Kennedy S, Kramer V, LeBlanc G, Mayes M, Nimrod A, Patino R, Peterson R, Purdy R, Ringer R, Thomas P, Touart L, van der Kraak G, Zacharewski T (1998) Overview of a workshop on screening methods for detecting potential (anti-) estrogenic/androgenic chemicals in wildlife. Environ Toxicol and Chem 17(1):68–87
Blaise C, Gagne F, Salazar M, Salazar S, Trottier S, Hansen PD (2003) Experimentally-induced feminisation of freshwater mussels after long-term exposure to a municipal effluent. Fresen Environ Bull 12(8):865–870
Cespedes R, Lacorte S, Raldua D, Ginebreda A, Barcelo D, Pina B (2005) Distribution of endocrine disruptors in the Llobregat river basin (Catalonia, NE Spain). Chemosphere 61(11):1710–1719
DIN 38415–6 (2003) Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammbehandlung—Suborganismische Testverfahren (Gruppe T)—Teil 6: Giftigkeit gegenüber Fischen, Bestimmung der nicht akut giftigen Wirkung von Abwasser auf die Entwicklung von Fischeiern über Verdünnungsstufen (T6)
Dizer H, Fischer B, Sepulveda L, Loffredo E, Senesi N, Santana F, Hansen PD (2002) Estrogenic effect of leachates and soil extracts from lysimeters spiked with sewage sludge and reference endocrine disruptors. Environ Toxicol 17(2):105–1112
Eroschenko VP, Palmiter RD (1980) Estrogenicity of kepone in birds and mammals. In: McLachlan JA (ed) Estrogens in the Environment. Elsevier, New York, pp 305–325
Fossi MC, Casini S, Marsili L (2006) Endocrine disruptors in Mediterranean top marine predators. Environ Sci Pollut Res 13(3):204–207
Fox GA (2001) Effects of endocrine disrupting chemicals on wildlife in Canada: past, present and future. Walter Qual Res J Canada 36(2):233–251
Gracia T, Jones PD, Higley EB, Hilscherova K, Newsted JL, Murphy MB, Chan AKY, Zhang X, Hecker M, Lam PKS, Wu RSS, Giesy JP (2008) Modulation of steroidogenesis by coastal waters and sewage effluents of Hong Kong, China using the H295R assay. Environ Sci Pollut Res 15(4):332–343
Guillette LJJ, Gross TS, Mason GR, Matter JM, Percival HF, Woodward AR (1994) Developmental abnormalities of the gonad and abnormal sex-hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect 102:680–688
Hecker M, Hollert H, Cooper R, Vinggard A-M, Akahori Y, Murphy M, Nellemann C, Higley E, Newsted, Wu R, Lam P, Laskey J, Buckalew A, Grund S, Nakai M, Timm G, Giesy J (2007) The OECD validation program of the H295R steroidgenesis assay for the identification of in vitro inhibitors or inducers of testosterone and estradiol production. Phase 2: inter laboratory pre-validation studies. Environ Sci Pollut Res 14(Spec Issue):23–30
Hollert H, Dürr M, Holtey-Weber R, Islinger M, Brack W, Färber H, Erdinger L, Braunbeck T (2005) Endocrine disruption of water and sediment extracts in a non-radioactive dot blot/RNAse protection-assay using isolated hepatocytes of rainbow trout. Environ Sci Pollut Res 12(6):347–360
Huschek G, Hansen P-D (2006) Ecotoxicological classification of the Berlin river system using bioassays in respect to the European Water Framework Directive. Environ Monit Assess 121(1–3):15–31
ISO 10253: (2006) Water quality—marine algal growth inhibition test with Skeletonema costatum and Phaeodactylum tricornutum
Kase R, Hansen P-D, Fischer B, Manz W, Heininger P, Reifferscheid G (2008) Integral assessment of estrogenic potentials of sediment-associated samples. Part 1: the influence of salinity on the in vitro tests ELRA, E-Screen and YES. Env Sci Pollut Res 15(1):75–83
Krebs F (2001) Ökotoxikologische Baggergutuntersuchung, Baggergutklassifizierung und Handhabungskategorien für Baggergut. In: Calmano W (ed) Untersuchung und Bewertung von Sedimenten. Springer, Heidelberg
Krebs F (2005) In: Blaise C, Ferard J-F (eds) The pT-method as a hazard assessment scheme for sediments and dredged material, Small-scale freshwater toxicity investigations. vol. 2. Springer, Heidelberg, pp 281–304
Länge R, Hutchinson TH, Croudace CP, Siegmund F, Schweinfurth H, Hampe P, Pante GH, Sumpter JP (2001) Effects of the synthetic estrogen 17-α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ Toxicol and Chem 20(6):1216–1227
Lutz I, Kloas W (1999) Amphibians as a model to study endocrine disruptors: l. Environmental pollution and estrogen receptor binding. Sci Total Environ 225(1–2):49–57
Lutz I, Kloas W, Einspanier R (1999) Amphibians as a model to study endocrine disruptors: II. estrogenic activity of environmental chemicals in vitro and in vivo. Sci Total Environ 225(1–2):59–68
Pane L, Giacco E, Corra C, Greco G, Mariotini GL, Varisco F, Faimali M (2008) Ecotoxicological evaluation of harbour sediments using organisms from different trophic levels. J Soils Sediments 8(2):74–79
Petermeier A, Schöll F, Tittizer T (1996) Die ökologische und biologische Entwicklung der deutschen Elbe. Lauterbornia 24, ISSN 0935–333X
Rastall AC, Getting D, Goddard J, Roberts DR Erdinger L (2006) A biomimetic approach to the detection and identification of estrogen receptor agonists in surface waters using semipermeable membrane devices (SPMDs) and bioassay-directed chemical analysis. Environ Sci Pollut Res 13(4):256–267
Reincke H, Löffler J, Wolff S, Bergemann M (1999):Wassergütedaten der Elbe Zahlentafel. ISSN 0931–2153
Rodbard D (1971) Statistical aspect of radioimmunoassay. In: Odell WD, Daughaday JB, Lipincott JB (eds) Principles of competitive protein binding assays. Lippincott Williams & Wilkins, Philadelphia, pp 204–259
Rodbard D, Hutt DM (1974) Statistical analysis of radioimmunoassays and immunoradiometric (labelled antibody) assay: a generalized weighted, iterative, least-squares method for logistic curve fitting. Radio-immunoassay and related procedures in Medicine 1:165–192
Sachs L (1999) Angewandte Statistik. Anwendung statistischer Methoden, 9. überarbeitete Auflage, Springer
Seibert H (1996) Störungen der Entwicklung und Funktion des männlichen Reproduktionssystems. Umweltwiss Schadst Forsch 8(5):275–284
Seifert M (1999) Bestimmung von Oestrogenen und Xenooestrogenen mit einem Rezeptorassay. Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften an der Technischen Universität München
Seifert M (2003) Luminescent enzyme linked receptor assay for estrogenic compounds. Anal Bioanal Chem 378(3):684–687
Seifert M, Haindl S, Hock B (1998) In vitro analysis of xenoestrogens byenzyme linked receptor assays (ELRA). Adv Exp Med Biol 444:113–117
Seifert M, Haindl S, Hock B (1999) Development of an enzyme linked receptor assay (ELRA) for estrogens and xenoestrogens. Elsevier: Anal Chim Acta 386:191–199
Stifani S, George R, Schneider WJ (1988) Solubilization and characterization of the chicken oocyte vitellogenin receptor. Biochem J 250:467–475
Voutsa D, Hartmann P, Schaffner C, Giger W (2006) Benzotriazoles, alkylphenols and bisphenol A in municipal wastewaters and in the Glatt River, Switzerland. Environ Sci Pollut Res 13(5):333–341
Acknowledgement
We are grateful for editorial support by Bernd Uebelmann, Federal Institute of Hydrology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jan Schwarzbauer
Rights and permissions
About this article
Cite this article
Kase, R., Hansen, P.D., Fischer, B. et al. Integral assessment of estrogenic potentials in sediment-associated samples. Environ Sci Pollut Res 16, 54–64 (2009). https://doi.org/10.1007/s11356-008-0060-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-008-0060-x