Abstract
Purpose
Uranium contamination of subsurface environments was once thought to be an isolated occurrence, mostly at production sites. But recent evidence has shown that the presence of uranium in phosphate fertilizers has caused massive amounts of this element to be released worldwide. Concerns are related to uranium movement to groundwater supplies and its significant toxicological risks to human populations. Information is direly needed on how geochemical processes control uranium transport in the vadose zone.
Materials and methods
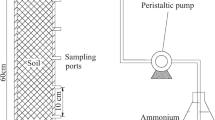
Laboratory experiments were performed to investigate the effects of the pH of the soil solution on the reactive transport of uranium(VI) in the vadose zone. The uranium solution was prepared by dilution of a 10−3 M stock solution of uranium perchlorate, (UO2(ClO4)2), with DI water. Two U(VI) solutions were prepared at concentrations of 2 × 10−6 M at pH 6 and 11 and were percolated under steady-state conditions through columns filled with sand. The convective-dispersion equation (CDE) was used to analyze the tracer and uranium breakthrough curves resulting from the column experiments. The program CXTFIT was used to estimate the transport parameters of equilibrium and nonequilibrium (i.e., two-site and mobile-immobile) models applied to the experimental data.
Results and discussion
Comparison of U(VI) breakthrough behavior at pH 6 with that of a nonreactive tracer indicated that U(VI) transport was significantly retarded, and about 52 % of the added U(VI) adsorbed to the quartz sand, likely in the cationic forms UO2OH+ and UO 2 +2 . The adsorption was reversible upon the addition of deionized water. At pH 11, the U(VI) breakthrough curve increased gradually and reached a plateau value C/C 0 oscillating between 72 and 82 %. Upon reaction, Si was released from the dissolution of quartz sand, which allowed the possible transport of U(VI) following precipitation of a U(VI) containing solid, such as uranyl-silicate minerals, or sorption of U(VI) onto silica colloids. Two-site and mobile-immobile (MIM) models suggested an influence of either rate-limited mass transfer processes or immobile/mobile water partitioning in U(VI) reactive transport.
Conclusions
The reactive transport of U(VI) governed by adsorption-desorption processes, precipitation, and complexation reactions in which kinetic behaviors are controlled by pH, solution chemistry, and heterogeneous flow regime impacts the mobility of U(VI). The column transport experiments indicated that under geochemical conditions and vadoze zone processes (preferential flow) that favor the mobility of U(VI), dissolved- and colloidal-phase associations of U(VI) may be transported rapidly and in high concentrations from the soil surface to the groundwater.



Similar content being viewed by others
References
Abdelouas A, Lutze W, Nuttall E (1998) Chemical reactions of uranium in ground water at a mill tailings site. J Contam Hydrol 34:343–361
Aharoni C, Sparks DL (1991) Kinetics of soil chemical processes – A theoretical treatment. In: Sparks DL, Suarez DL (eds) Rate of soil chemical processes. Soil Sci Soc Am Spec Publ 27, Soil Sci Soc Am Inc, Madison, WI, pp 1–18
Andersson K, Torstenfelt B, Allard B (1982) Sorption behavior of long-lived radionuclides in igneous rock. In: International Atomic Energy Agency, the Commission of the European Communities and the OECD Nuclear Energy Agency (ed) Environmental migration of long-lived radionuclides, Vienna, Austria, pp 111–131
Arai Y, Marcus MK, Tamura N, Davis JA, Zachara JM (2007) Spectroscopic evidence for uranium bearing precipitates in vadose zone sediments at the Hanford 300-area site. Environ Sci Technol 41:4633–4639
Arnold T, Zorn T, Bernhard G, Nitsche H (1998) Sorption of uranium(VI) onto phyllite. Chem Geol 151:129–141
Arnold T, Zorn T, Zänker H, Bernhard G, Nitsche H (2001) Sorption behavior of U(VI) on phyllite: experiments and modeling. J Contam Hydrol 47:219–231
Artinger R, Rabung T, Kim JI, Sachs S, Schmeide K, Heise KH, Bernhard G, Nitsche H (2002) Humic colloid-borne migration of uranium in sand columns. J Contam Hydrol 58:1–12
Baik MH, Hahn PS (2001) Experimental study on uranium sorption onto silica colloids: effects of geochemical parameters. J Korean Nucl Soc 33:261–269
Ball MC, Brown DS, Perkins LJ (1997) Diffuse layers on the surface of mineral quartz. J Mater Chem 7:365–368
Bargar JR, Reitmeyer R, Lenhart JJ, Davis JA (2000) Characterization of U(VI)-carbonato ternary complexes on hematite: EXAFS and electrophoretic mobility measurements. Geochim Cosmochim Acta 64:2737–2749
Barnett MO, Jardine PM, Brooks SC, Selim HM (2000) Adsorption and transport of uranium(VI) in subsurface media. Soil Sci Soc Am J 64:908–917
Bethke CM (1996) Geochemical reaction modeling. Concepts and applications. Oxford University Press, New York
Bethke CM (2002) The Geochemist’s Workbench® 4.0. University of Illinois, Urbana
Bickmore BR, Nagy KL, Young JS, Drexler JW (2001) Nitrate-cancrinite precipitation on quartz sand in simulated Hanford tank solutions. Environ Sci Technol 35:4481–4486
Bickmore BR, Nagy KL, Gray AK, Brinkerhoff AR (2006) The effect of Al(OH)4− on the dissolution rate of quartz. Geochim Cosmochim Acta 70:290–305
Cameron DA, Klute A (1977) Convective-dispersive solute transport with a combined equilibrium and kinetic adsorption model. Water Resour Res 19:718–724
Catalano JG, McKinley JP, Zachara JM, Heald SM, Smith SC, Brown GE (2006) Changes in uranium speciation through a depth sequence of contaminated Hanford sediments. Environ Sci Technol 40:2517–2524
Chen G, Flury M (2005) Retention of mineral colloids in unsaturated porous media as related to their surface properties. Colloids Surf A Physicochem Eng Asp 256:207–216
Cheng T, Barnett MO, Roden EE, Zhuang J (2007) Reactive transport of uranium(VI) and phosphate in a goethite-coated sand column: an experimental study. Chemosphere 68:1218–1223
Coats KH, Smith BD (1964) Dead-end pore volume and dispersion in porous media. Soc Petrol Eng J 4:73–84
Contardi JS, Turner DR, Ahn TM (2001) Modeling colloid transport for performance assessment. J Contam Hydrol 47:323–333
Crançon P, van der Lee J (2003) Speciation and mobility of uranium(VI) in humic-containing soils. Radiochim Acta 91:673–9
Crançon P, Pili E, Charlet L (2010) Uranium facilitated transport by water-dispersible colloids in field and soil columns. Sci Total Environ 408:2118–2128
CXTFIT/EXCEL (2013) CXTFIT code in excel. http://web.ornl.gov/~t6g/cxtfit/ (accessed 26 July 2014)
Fortner JA, Mertz CJ, Goldberg MM, Seifert S (2002) Characteristics of aqueous colloids generated by corrosion of metallic uranium fuel. ANL/CMT/CP-106586. Argonne National Laboratory, Argonne. http://www.ipd.anl.gov/anlpubs/2002/09/44366.pdf. Accessed 13 June 2013
Fox PM, Davis JA, Zachara JM (2006) The effect of calcium on aqueous uranium(VI) speciation and adsorption to ferrihydrite and quartz. Geochim Cosmochim Acta 70:1379–1387
Gabriel U, Gaudet J-P, Spadini L, Charlet L (1998) Reactive transport of uranyl in agoethite column: an experimental and modelling study. Chem Geol 151:107–128
Gamerdinger AP, Kaplan DI, Wellman DM, Serne RJ (2001a) Two-region flow and rate-limited sorption of uranium (VI) during transport in unsaturated silt loam. Water Resour Res 37:3147–3153
Gamerdinger AP, Kaplan DI, Wellman DM, Serne RJ (2001b) Two-region flow and decreased sorption of uranium (VI) during transport in Hanford groundwater and unsaturated sands. Water Resour Res 37:3155–3162
Giblin AM (1980) The role of clay adsorption in genesis of uranium ores. In: Ferguson J, Goleby AB (eds) Uranium in the Pine Creek geosyncline. International Atomic Energy Agency, Vienna, pp 521–529
Handley-Sidhu S, Bryan ND, Worsfold PJ, Vaughan DJ, Livens FR, Keith-Roach MJ (2009) Corrosion and transport of depleted uranium in sand-rich environments. Chemosphere 77:1434–1439
Ho CH, Miller NH (1986) Adsorption of uranyl species from bicarbonate solution onto hematite particles. J Colloid Interface Sci 110:165–171
Huber F, Lützenkirchen J (2009) Uranyl retention on quartz—new experimental data and blind prediction using an existing surface complexation model. Aquat Geochem 15:443–456
Jada A, Ait Akbour R, Douch J (2006) Surface charge and adsorption from water onto quartz sand of humic acid. Chemosphere 64:1287–1295
Jones TE, Wood MI, Corbin RA, Simpson BC (2001) Preliminary inventory estimates for single-shell tank leaks in B, BX, and BY tank farms. RPP-7389. CH2M Hill Hanford Group, Inc, Richland
Kaplan DI, Serne RJ (1995) Distribution coefficient values describing iodine, neptunium, selenium, technetium, and uranium sorption to Hanford sediments. PNL-10379, SUP. 1. Pacific Northwest Laboratory, Richland
Kaplan DI, Gervais TL, Krupka KM (1998) Uranium(VI) sorption to sediments under high pH and ionic strength conditions. Radiochim Acta 80:201–211
Ku TL, Luo S, Goldstein SJ, Murrell MT, Chu WL, Dobson PF (2009) Modeling non-steady state radioisotope transport in the vadose zone—a case study using uranium isotopes at Peña Blanca, Mexico. Geochim Cosmochim Acta 73:6052–6064
Larson LN, Stone JJ (2011) Sediment-bound arsenic and uranium within the Bowman-Haley Reservoir, North Dakota. Water Air Soil Pollut 219:27–42
Leduc LG, Ferroni GD, Trevors JT (1997) Resistance to heavy metals in different strains of Thiobacillus ferrooxidans. World J Microbiol Biotechnol 13:453–455
Lenhart JJ, Honeyman BD (1999) Uranium(VI) sorption to hematite in the presence of humic acid. Geochim Cosmochim Acta 63:2891–901
Lesher EK, Honeyman BD, Ranville JF (2013) Detection and characterization of uranium-humic complexes during 1D transport studies. Geochim Cosmochim Acta 109:127–142
Liu C, Zachara JM, Qafoku O, McKinley JP, Heald SM, Wang Z (2004) Dissolution of uranyl microprecipitates in subsurface sediments at Hanford site, USA. Geochim Cosmochim Acta 68:4519–4537
Liu CX, Zachara JM, Yantasee W, Majors PD, McKinley JP (2006) Microscopic reactive diffusion of uranium in the contaminated sediments at Hanford, United States. Water Resour Res 42:15
Liu C, Shi Z, Zachara JM (2009) Kinetics of uranium(VI) desorption from contaminated sediments: effect of geochemical conditions and model evaluation. Environ Sci Technol 43:6560–6566
Lu L, Conca J, Parker GR, Leonard PA, Moore B, Strietelmeier B, Triay IR (2000) Adsorption of actinides onto colloids as a function of time, temperature, ionic strength, and colloid concentration. Report LA-UR- 00-51-21. Los Alamos National Laboratory, Los Alamos
Ma R, Zheng C, Prommer H, Greskowiak J, Liu C, Zachara J, Rockhold M (2010) A field-scale reactive transport model for U(VI) migration influenced by coupled multirate mass transfer and surface complexation reactions. Water Resour Res. doi:10.1029/2009WR008168
Ma R, Zheng C, Liu C, Greskowiak J, Prommer H, Zachara J (2014a) Assessment of controlling processes for field-scale uranium reactive transport under highly transient flow conditions. Water Resour Res 50:1006–1024
Ma R, Liu C, Greskowiak J, Prommer H, Zachara J, Zheng C (2014b) Influence of calcite on uranium(VI) reactive transport in the groundwater-river mixing zone. J Contam Hydrol 156:27–37
Malin JN, Geiger FM (2010) Uranyl adsorption and speciation at the fused silica/water interface studied by resonantly enhanced second harmonic generation and the χ3 method. J Phys Chem A 114:1797–1805
Mashal K, Harsh JB, Flury M, Felmy AR, Zhaq H (2004) Colloid formation in Hanford sediments reacted with simulated tank waste. Environ Sci Technol 38:5750–5756
McKinley JP, Zachara JM, Liu C, Heald SC, Prenitzer BI, Kempshall BW (2006) Microscale controls on the fate of contaminant uranium in the vadose zone, Hanford site, Washington. Geochim Cosmochim Acta 70:1873–1887
McKinley JP, Zachara JM, Wan J, McCready DE, Heald SM (2007a) Geochemical controls on contaminant uranium in vadose Hanford formation sediments at the 200 area and 300 area, Hanford site, Washington. Vadose Zone J 6:1004–1017
McKinley JP, Zachara JM, Smith SC, Liu C (2007b) Cation exchange reactions controlling desorption of 90Sr2+ from coarse-grained contaminated sediments at the Hanford site, Washington. Geochim Cosmochim Acta 71:305–325
Mertz CJ, Fortner JA, Tsai Y (2003) Understanding the behavior and stability of some uranium mineral colloids. In: Finch RJ, Bullen DB (eds) Scientific basis for nuclear waste management XXVI, vol 757. Materials Research Society Symposium Proceedings, Warrendale, pp 489–496
Mibus J, Sachs S, Pfingsten W, Nebelung C, Bernhard G (2007) Migration of uranium(IV)/(VI) in the presence of humic acids in quartz sand: a laboratory column study. J Contam Hydrol 89:199–217
Mihalik J, Tlustos P, Szakova J (2011) The impact of an abandoned uranium mining area on the contamination of agricultural land in its surroundings. Water Air Soil Pollut 215:693–700
Miller AW, Rodriguez DR, Honeyman BD (2013a) Simplified behaviors from increased heterogeneity: I. 2-D uranium transport experiments at the decimeter scale. J Contam Hydrol 148:39–50
Miller AW, Rodriguez DR, Honeyman BD (2013b) Simplified behaviors from increased heterogeneity: II. 3-D uranium transport at the decimeter scale and intertank comparisons. J Contam Hydrol 148:51–66
Missana T, García-Gutiérrez M, Alonso Ú (2004) Kinetics and irreversibility of cesium and uranium sorption onto bentonite colloids in a deep granitic environment. Appl Clay Sci 26:137–150
Moyes LN, Parkman RH, Charnock JM, Vaughan DJ, Livens FR, Hughes CR, Braithwaite A (2000) Uranium uptake from aqueous solution by interaction with goethite, lepidocrocite, muscovite, and mackinawite: an X-ray absorption spectroscopy study. Environ Sci Technol 34:1062–1068
Nielsen DR, van Genuchten MT, Biggar JW (1986) Water flow and solute transport processes in the unsaturated zone. Water Resour Res 22:89S–108S
Nkedi-Kizza P, Biggar JW, Selim HM, van Genuchten MT, Wierenga PJ, Davidson JM, Nielsen DR (1984) On the equivalence of two conceptual models for describing ion exchange during transport through an aggregated oxisol. Water Resour Res 20:1123–1130
Parker JC, van Genuchten MTh (1984) Determining transport parameters from laboratory and field tracer experiments. Bull 84–3, Va Agric Exp St, Blacksburg
Phillippi JM, Loganathan VA, McIndoe MJ, Barnett MO, Clement TP, Roden EE (2007) Theoretical solid/solution ratio effects on adsorption and transport: uranium(VI) and carbonate. Soil Sci Soc Am J 71:329–335
Prikryl JD, Jain A, Turner DR, Pabalan RT (2001) UraniumVI sorption behavior on silicate mineral mixtures. J Contam Hydrol 47:241–253
Qafoku NP, Ainsworth CC, Szecsody JE, Qafoku OS (2004) Transport-controlled kinetics of dissolution and precipitation in the sediments under alkaline and saline conditions. Geochim Cosmochim Acta 68:2981–2995
Qafoku NP, Zachara JM, Liu C, Gassman PL, Qafoku OS, Smith SC (2005) Kinetic desorption and sorption of U(VI) during reactive transport in a contaminated Hanford sediment. Environ Sci Technol 39:3157–3165
Read D, Lawless TA, Sims RJ, Butter KR (1993) Uranium migration through intact sandstone cores. J Contam Hydrol 13:277–289
Redden G, Bargar J, Bencheikh-Latmani R (2001) Citrate enhanced uranyl adsorption on goethite: an EXAFS analysis. J Colloid Interface Sci 244:211–219
Reich T, Moll H, Arnold T, Denecke MA, Hennig C, Geipel G, Bernhard G, Nitsche H, Allen PG, Bucher JJ, Edelstein NM, Shuh DK (1998) An EXAFS study of uranium(VI) sorption onto silica gel and ferrihydrite. J Electron Spectrosc Relat Phenom 96:237–243
Rimstidt JD, Barnes HL (1980) The kinetics of silica-water reactions. Geochim Cosmochim Acta 44:1683–1699
Rod KA, Um W, Flury M (2010) Transport of strontium and cesium in simulated Hanford tank waste leachate through quartz sand under saturated and unsaturated flow. Environ Sci Technol 44:8089–8094
Rod KA, Wellman DM, Flury M, Pierce EM, Harsh JB (2012) Diffuse release of uranium from contaminated sediments into capillary fringe pore water. J Contam Hydrol 140–141:164–172
Salter PF, Ames LL, McGarrah JE (1981) The sorption behavior of selected radionuclides on Columbia River basalts, RHO-BWI-LD-48. Rockwell Hanford Operations, Richland
Schnug E, Lottermoser BG (2013) Fertilizer-derived uranium and its threat to human health. Environ Sci Technol 47:2433–2434
Selim HM, Davidson JM, Mansell RS (1976) Evaluation of a two-site adsorption-desorption model for describing solute transport in soils. In Proc Summer Computer Simulation Conf, Washington, DC
Serne RJ, Clayton RE, Kutnyakov IV, Last GV, LeGore VL, Wilson TC, Schaef HT, O’Hara MJ, Wagnon KB, Lanigan DC, Brown CF, Williams BA, Lindenmeier CW, Orr RD, Burke DS, Ainsworth CC (2002) Characterization of vadose zone sediment: borehole 41-09-39 in the S-SX waste management area, PNNL-13757-3. U.S. Department of Energy, Pacific Northwest National Laboratory, Richland
Shang J, Liu C, Wang Z, Zachara JM (2011) Effect of grain size on uranium(VI) surface complexation kinetics and adsorption additivity. Environ Sci Technol 45:6025–6031
Shang J, Liu C, Wang Z, Zachara J (2014) Long-term kinetics of uranyl desorption from sediments under advective conditions. Water Resour Res 50:855–870
Sheppard MI, Thibault DH (1988) Migration of technetium, iodine, neptunium, and uranium in the peat of two minerotrophic mires. J Environ Qual 17:644–653
Sims R, Lawless TA, Alexander JL, Bennett DG, Read D (1996) Uranium migration through intact sandstone: effect of pollutant concentration and the reversibility of uptake. J Contam Hydrol 21:215–228
Stoliker DL, Kent DB, Zachara JM (2011) Quantifying differences in the impact of variable chemistry on equilibrium uranium(VI) adsorption properties of aquifer sediments. Environ Sci Technol 45:8733–8740
Stoliker DL, Liu C, Kent DB, Zachara JM (2013) Characterizing particle-scale equilibrium adsorption and kinetics of uranium(VI) desorption from U-contaminated sediments. Water Resour Res 49:1163–1177
Stubbs JE, Veblen LA, Elbert DC, Zachara JM, Davis JA, Veblen DR (2009) Newly recognized hosts for uranium in the Hanford Site vadose zone. Geochim Cosmochim Acta 73:1563–1576
Tang G, Mayes MA, Parker JC, Jardine PM (2010) CXTFIT/Excel—a modular adaptable code for parameter estimation, sensitivity analysis and uncertainty analysis for laboratory or field tracer experiments. Comput Geosci 36:1200–1209
Tompson AFB, Hudson GB, Smith DK, Hunt JR (2006) Analysis of radionuclide migration through a 200-m vadose zone following a 16-year infiltration event. Adv Water Resour 29:281–292
Toride N, Leij FJ, van Genuchten MT (1999) The CXTFIT code for estimating transport parameters from laboratory or field tracer experiments. Version 2.1, Research Report No. 137. U.S. Salinity Laboratory, U.S. Department of Agriculture, Riverside
Um W, Wang Z, Serne RJ, Williams BD, Brown CF, Dodge CJ, Francis AJ (2009) Uranium phases in contaminated sediments below Hanford’s U tank farm. Environ Sci Technol 43:4280–4286
Um W, Icenhower JP, Brown CF, Serne RJ, Wang Z, Dodge CJ, Francis AJ (2010a) Characterization of uranium-contaminated sediments from beneath a nuclear waste storage tank from Hanford, Washington: implications for contaminant transport and fate. Geochim Cosmochim Acta 74:1363–1380
Um W, Zachara JM, Liu C, Moore DA, Rod KA (2010b) Resupply mechanism to a contaminated aquifer: a laboratory study of U(VI) desorption from capillary fringe sediments. Geochim Cosmochim Acta 74:5155–5170
US EPA (U.S. Environmental Protection Agency) (1999) Understanding variation in partition coefficient, Kd, values: volume II. Review of geochemistry and available Kd values for cadmium, cesium, chromium, lead, plutonium, radon, strontium, thorium, tritium (3H), and uranium. U.S. Environmental Protection Agency, Office of Air and Radiation. EPA 402-R-99-004B http://www.epa.gov/radiation/docs/kdreport/vol2/402-r-99-004b.pdf. Accessed 12 June 2013
van Genuchten MT, Wagenet RJ (1989) Two-site/two-region models for pesticide transport and degradation: theoretical development and analytical solutions. Soil Sci Soc Am J 53:1303–1310
van Genuchten MT, Wierenga PJ (1976) Mass transfer studies in sorbing porous media: I. Analytical solutions. Soil Sci Soc Am J 40:473–481
Viani BE, Torretto PC (1998) Sorption and transport of uranium on hematite. UCRL-ID-129848, YMP Milestone Report SPL3BM4 - 1997. Lawrence Livermore National Laboratory, Livermore
Waite TD, Davis JA, Payne TE, Waychunas GA, Xu N (1994) Uranium(VI) adsorption to ferrihydrite: application of a surface complexation model. Geochim Cosmochim Acta 58:5465–5478
Wang G, Um W (2012) Mineral dissolution and secondary precipitation on quartz sand in simulated Hanford tank solutions affecting subsurface porosity. J Hydrol 472–473:159–168
Wang Z, Zachara JM, Gassman PL, Liu C, Qafoku O, Yantasee W, Catalano JG (2005) Fluorescence spectroscopy of U(VI)-silicates and U(VI)-contaminated Hanford sediment. Geochim Cosmochim Acta 69:1391–1403
Wellman DM, Gamerdinger AP, Kaplan DI, Serne RJ (2008) Effect of particle-scale heterogeneity on uranium(VI) transport in unsaturated porous media. Vadose Zone J 7:67–78
Williams BA, Brown CF, Um W, Nimmons MJ, Peterson RE, Bjornstad BN, Lanigan DC, Serne RJ, Spane FA, Rockhold ML (2007) Limited field investigation report for uranium contamination in the 300 area, 300-FF-5 operable unit, Hanford Site, Washington. PNNL-16435. Pacific Northwest National Laboratory, Richland
Yabusaki SB, Fang Y, Waichler SR (2008) Building conceptual models of field-scale uranium reactive transport in a dynamic vadose zone-aquifer-river system. Water Resour Res. doi:10.1029/2007WR006617
Yabusaki SB, Fang Y, Williams KH, Murray CJ, Ward AL, Dayvault RD, Waichler SR, Newcomer DR, Spane FA, Long PE (2011) Variably saturated flow and multicomponent biogeochemical reactive transport modeling of a uranium bioremediation field experiment. J Contam Hydrol 126:271–290
Yin J, Haggerty R, Stoliker DL, Kent DB, Istok JD, Greskowiak J, Zachara JM (2011) Transient groundwater chemistry near a river: effects on U(VI) transport in laboratory column experiments. Water Resour Res. doi:10.1029/2010WR009369
Zachara JM, Ainsworth CC, McKinley JP, Murphy EM, Westall JC, Rao PSC (1992) Subsurface chemistry of organic ligand-radionuclide mixtures. In: Pacific Northwest Laboratory Annual Report for 1991 to the DOE Office of Energy Research Part 2: Environmental Science, PNL-8000 Pt. 2. Pacific Northwest Laboratory, Richland, pp 1–12
Zänker H, Hüttig G, Arnold T, Nitsche H (2006) Formation of iron-containing colloids by the weathering of phyllite. Aquat Geochem 12:299–325
Zheng Z, Wan J (2005) Release of contaminant U(VI) from soils. Radiochim Acta 93:211–217
Zhuang J, Flury M, Jin Y (2003) Colloid-facilitated Cs transport through water-saturated Hanford sediment and Ottawa sand. Environ Sci Technol 37:4905–4911
Zielinski RA (1980) Uranium in secondary silica: a possible exploration guide. Econ Geol 75:592–602
Acknowledgments
This research was supported by the U.S. Department of Energy, Office of Biological and Environmental Research, Environmental Remediation Sciences Program under grant number DE-FG02-06ER64193, and Clemson University. We thank Dr. Zhihong Xu, Editor-in-Chief of the Journal of Soils and Sediments, and the two anonymous reviewers for their thoughtful and constructive comments to improve our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Dong-Mei Zhou
Rights and permissions
About this article
Cite this article
Uyuşur, B., Li, C., Baveye, P.C. et al. pH-dependent reactive transport of uranium(VI) in unsaturated sand. J Soils Sediments 15, 634–647 (2015). https://doi.org/10.1007/s11368-014-1018-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-1018-x