Abstract
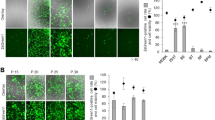
Embryo-derived cell lines are important in vitro models for investigating the molecular mechanisms directing embryonic tissue lineage segregation and maintenance. The bovine trophectoderm-derived CT-1 cell line has been widely used to identify regulatory mechanisms of interferon tau gene expression, and it possesses potential as a model for characterizing the gene regulatory network controlling trophoblast lineage differentiation and development. This functional potential, however, is severely limited as CT-1 cells are very recalcitrant to standard transfection methods. The focus of this study was to test the cationic lipitoid reagent as an effective transfection reagent for DNA plasmid delivery. Optimization of liptoid-based transfection of plasmid DNA resulted in 9% transfection efficiency averaged across entire CT-1 colonies, with many subregions of CT-1 colonies achieving transfection rates of 15%. These rates are a substantial improvement over near-zero efficiencies achieved using other standard transfection techniques. CT-1 cells were also successfully adapted to substrate-free culture for over 20 passages, eliminating the need to culture CT-1 colonies on feeder cells or matrix-coated cultureware. Together, these results increase the utility of the CT-1 cell line as an in vitro bovine trophoblast model and provide insight into overcoming DNA delivery difficulties in other cell lines not amenable to genetic manipulation.


Similar content being viewed by others
References
Bai H.; Sakurai T.; Kim M. S.; Muroi Y.; Ideta A.; Aoyagi Y.; Nakajima H.; Takahashi M.; Nagaoka K.; Imakawa K. Involvement of GATA transcription factors in the regulation of endogenous bovine interferon-tau gene transcription. Mol. Reprod. Dev. 76(12): 1143–1152; 2009.
Bai H.; Sakurai T.; Someya Y.; Konno T.; Ideta A.; Aoyagi Y.; Imakawa K. Regulation of trophoblast-specific factors by GATA2 and GATA3 in bovine trophoblast CT-1 cells. J. Reprod. Dev. 57(4): 518–525; 2011.
Berg D. K.; Smith C. S.; Pearton D. J.; Wells D. N.; Broadhurst R.; Donnison M.; Pfeffer P. L. Trophectoderm lineage determination in cattle. Dev. Cell 20(2): 244–255; 2011.
Degrelle S. A.; Campion E.; Cabau C.; Piumi F.; Reinaud P.; Richard C.; Renard J. P.; Hue I. Molecular evidence for a critical period in mural trophoblast development in bovine blastocysts. Dev. Biol. 288(2): 448–460; 2005.
Ezashi T.; Ghosh D.; Roberts R. M. Repression of Ets-2-induced transactivation of the tau interferon promoter by Oct-4. Mol. Cell. Biol. 21(23): 7883–7891; 2001.
Huang C. Y.; Uno T.; Murphy J. E.; Lee S.; Hamer J. D.; Escobedo J. A.; Cohen F. E.; Radhakrishnan R.; Dwarki V.; Zuckermann R. N. Lipitoids-novel cationic lipids for cellular delivery of plasmid DNA in vitro. Chem. Biol. 5(6): 345–354; 1998.
Michael D. D.; Alvarez I. M.; Ocon O. M.; Powell A. M.; Talbot N. C.; Johnson S. E.; Ealy A. D. Fibroblast growth factor-2 is expressed by the bovine uterus and stimulates interferon-tau production in bovine trophectoderm. Endocrinology 147(7): 3571–3579; 2006a.
Michael D. D.; Wagner S. K.; Ocon O. M.; Talbot N. C.; Rooke J. A.; Ealy A. D. Granulocyte-macrophage colony-stimulating-factor increases interferon-tau protein secretion in bovine trophectoderm cells. Am. J. Reprod. Immunol. 56(1): 63–67; 2006b.
Pant D.; Keefer C. L. Expression of pluripotency-related genes during bovine inner cell mass explant culture. Cloning Stem Cells 11(3): 355–365; 2009.
Rossant J. Stem cells and lineage development in the mammalian blastocyst. Reprod. Fertil. Dev. 19(1): 111–118; 2007.
Sakurai T.; Sakamoto A.; Muroi Y.; Bai H.; Nagaoka K.; Tamura K.; Takahashi T.; Hashizume K.; Sakatani M.; Takahashi M.; Godkin J. D.; Imakawa K. Induction of endogenous interferon tau gene transcription by CDX2 and high acetylation in bovine nontrophoblast cells. Biol. Reprod. 80(6): 1223–1231; 2009.
Talbot N. C.; Caperna T. J.; Edwards J. L.; Garrett W.; Wells K. D.; Ealy A. D. Bovine blastocyst-derived trophectoderm and endoderm cell cultures: interferon tau and transferrin expression as respective in vitro markers. Biol. Reprod. 62(2): 235–247; 2000.
Talbot N. C.; Powell A. M.; Camp M.; Ealy A. D. Establishment of a bovine blastocyst-derived cell line collection for the comparative analysis of embryos created in vivo and by in vitro fertilization, somatic cell nuclear transfer, or parthenogenetic activation. In Vitro Cell. Dev. Biol. Anim. 43(2): 59–71; 2007.
Utku Y.; Dehan E.; Ouerfelli O.; Piano F.; Zuckermann R. N.; Pagano M.; Kirshenbaum K. A peptidomimetic siRNA transfection reagent for highly effective gene silencing. Mol. Biosyst. 2(6–7): 312–317; 2006.
Walker A. M.; Kimura K.; Roberts R. M. Expression of bovine interferon-tau variants according to sex and age of conceptuses. Theriogenology 72(1): 44–53; 2009.
Wells K. D.; Powell A. M. Blastomeres from somatic cell nuclear transfer embryos are not allocated randomly in chimeric blastocysts. Cloning 2(1): 9–22; 2000.
Yang Q. E.; Johnson S. E.; Ealy A. D. Protein kinase C delta mediates fibroblast growth factor-2-induced interferon-tau expression in bovine trophoblast. Biol. Reprod. 84(5): 933–943; 2011.
Zhang M.; Guller S.; Huang Y. Method to enhance transfection efficiency of cell lines and placental fibroblasts. Placenta 28(8–9): 779–782; 2007.
Acknowledgments
We thank Dr. Neil Talbot at the USDA (Beltsville, MD) for providing the phEFnGFP plasmid and CT-1 cells and also for his assistance in establishing CT-1 cultures. We thank Dr. Ronald Zuckermann of the Molecular Foundry of the Lawrence Berkeley National Laboratory, Berkeley, CA, for generously supplying lipitoid reagent. This research was supported by Maryland Agricultural Experiment Station grants.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Schiffmacher, A.T., Keefer, C.L. Optimization of a lipitoid-based plasmid DNA transfection protocol for bovine trophectoderm CT-1 cells. In Vitro Cell.Dev.Biol.-Animal 48, 403–406 (2012). https://doi.org/10.1007/s11626-012-9525-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-012-9525-9