Abstract
Ships’ hulls are typically coated with coating systems which have both anticorrosive and antifouling purposes, and consist of a primer, a tie coat, and an antifouling coat. Within this study, the protective properties of two different anticorrosion–antifouling coating systems are evaluated in a humid and warm atmosphere, as well as in a simulated marine environment utilizing salt spray chamber testing and immersion tests with and without agitation. The tested coating systems differ in the type of antifouling coating, i.e., self-polishing antifouling based on copper, and a new fouling-release coating based on miscellaneous components. The corrosion performances of Stojanović coatings in natural seawater are examined using open circuit potential measurements, electrochemical impedance spectroscopy, and pull-off tests. The antifouling properties are reviewed by visual examination, measurements of coating roughness before and after exposure to the testing solution, and after immersion in a solution containing Saccharomyces cerevisiae by measuring the optical density. The obtained results show better corrosion and antifouling properties of the coating system with a self-polishing antifouling top coat compared to the coating system with the foul-release top coat. A thicker biofilm was noticed on the plates immersed in seawater with agitation compared to stationary conditions for both antifouling coatings.
Similar content being viewed by others
Introduction
A ship’s hull coating system consists of a primer which has anticorrosion (AC) properties, a tie coat which provides a transition from the primer to the antifouling (AF) coat and which bonds generically different types of coatings, and an antifouling coating. Many parameters can have an influence on the performance of AC–AF coating system, which represents a functional barrier between the steel surface and the seawater, and regulates the diffusion and migration of seawater and other chemical species to the coating system and substrate interface. When the ship’s hull is immersed, it is not only subjected to corrosion but also to biofouling. The corrosion can lead to enormous costs and hull damage and thus significantly affect the ship’s structural safety. Corrosion increases the general stress level as the dimensions of the ship’s structural elements are reduced. Also, it produces cracking damage in welded joints, which are then subjected to fatigue cracking. In addition, the mechanical properties of corroded steel are significantly reduced compared to intact steel.1 Biofouling can be described as the accumulation of microorganisms, algae, plants or animals on wetted surfaces. It causes an increase of resistance and consequently increased fuel consumption, engine stress and greenhouse gas (GHG) emissions if the ship’s speed is kept constant.2,3 In order to prevent biofouling on ships’ hulls, AF coatings are most commonly applied. It should be noted that there are alternative methods for the prevention of biofouling which include aeration and acoustic methods, but they are still not widely applied.4,5 Nowadays, there are two main approaches in biofouling prevention with AF coatings, i.e., biocidal and non-biocidal coatings.6 Biocidal coatings slowly leach the incorporated biocides into the fluid and thus deter microorganisms from attaching to the surface.7 Traditionally, most AF coatings contained tributyltin (TBT) or copper as a biocide. In the 1980s, it was thought that the biofouling problem had been solved with the use of TBT. However, due to the detrimental effect of TBT on the environment, AF coatings based on TBT were forbidden by the International Maritime Organization in 2001 and banned worldwide in 2008. Therefore, presently, biocide agents are based on copper, but it is thought that these AF coatings will also be banned due to environmental concerns.8 For that reason, the emphasis is now being put on the development of non-toxic biocide-free coatings.9 One of the greatest efforts towards this path was carried out through a European Union research project entitled Advanced Nanostructured Surfaces for the Control of Biofouling (AMBIO).10 The goal of AMBIO was to develop novel coatings which would prevent or reduce the adhesion of biofouling through nanostructuring in order to obtain the desired physical and chemical properties of the surface instead of using biocides. Recently, there have been many studies related to environmentally friendly AF coatings. Peres et al.11 have studied the AF performance and water uptake behavior of coatings which had papain adsorbed in activated carbon as a pigment and rosin as a matrix. Electrochemical impedance spectroscopy (EIS) was used for the evaluation of the performance of the proposed coatings in the marine environment. Also, EIS was used to determine the apparent water coefficient of diffusion. The results confirmed the outstanding performance of papain-based AF coatings since the results were similar to those obtained with commercial coatings, taking into account that negative environmental concerns were avoided. De-doped bromo-substituted polyaniline was proven to have excellent antifouling and anticorrosion abilities using EIS and antibacterial tests,12 unlike other commonly used AF agents. Furthermore, this AF agent has a good anticorrosion ability even if the coating was damaged, and thus new insights into high-performance dual functional coatings were achieved. Hecker et al.13 have reported modifications of a polystyrene coating surface with the goal of inhibiting protein adsorption and achieving long-term protein rejection.
In addition to having a negative impact on the ship’s resistance, fuel consumption and GHG emissions, biofouling can significantly accelerate corrosion.14 Electrochemical analysis is commonly used for measuring the coating integrity and characteristics of corrosion resistance.15 In Ref. 16, the authors have shown that the coupling of EIS and open circuit potential (OCP) can be useful for the assessment of the functionality of AF coatings. The stability of the investigated system can be determined using OCP measurements, while EIS can be used to identify processes which cannot be identified using other techniques.17,18 Kiosidou et al.19 have investigated the barrier properties of two AF coatings, i.e., the fouling release (FR) coating based on silicone and the epoxy self-polishing coating using accelerated and natural weathering methods. The authors confirmed that the silicone FR coating had better AF protection than the self-polishing coating, acceptable barrier properties, and had retained color and gloss during the performed experiments. Wang et al.20 showed very good corrosion resistance properties of a composite epoxy coating with a modified graphene oxide synergistic structure using EIS. The anticorrosive and AF properties of organic coatings based on copper oxide were studied using EIS and scanning electron microscopy (SEM).21 The authors incorporated microbial exopolysaccharides (EPS) to investigate its influence on the corrosion behavior. However, since there was no adhesion, incorporation of EPS had no influence on anticorrosion properties. In Ref. 22, the authors investigated the surface morphology and corrosion performance of polyester and epoxy-polyester coatings using EIS and OCP measurements as well as SEM and energy-dispersive x-ray spectroscopy. The study showed a negative effect of coating surface texture on coating thickness, and thus a lack of barrier and adhesion properties.
Within this paper, the protective properties of two different AC–AF coatings were evaluated. These coatings differed in the applied AF coating, that is a biocide-based AF coating and a biocide-free AF coating. The biocide-based AF is self-polishing, chemically hydrolyzing antifouling based on nano-acrylate technology, and has been developed for vessels requiring protection for 60 months of service in worldwide waters. It contains copper as a biocide which is slowly leached into seawater, thus preventing the fouling of the ship’s hull. The leaching of the biocide is achieved by the hydrolysis process of the polymer and erosion. Copper as a biocide is less toxic to humans and much safer than TBT for non-target species, but still has a harmful effect.16,23 The second AF tested is a new biocide-free coating containing miscellaneous components, chosen for testing as an answer to environmental concerns due to heavy metal release in the environment.8,9 According to Ref. 24, antifouling systems that do not use heavy metals are the foul-release coatings. These coatings makes it difficult for fouling to fully attach, thus making it easy to be removed during navigation or by simple mechanical cleaning. Its binder technology controls the self-renewing effect throughout the season. The protective properties were tested in a humid and warm atmosphere, as well as in a simulated marine environment using salt spray chamber testing, and by the immersion in seawater in stationary and agitating conditions followed by EIS and OCP measurements. The roughness of the coatings was measured before and after the exposure to the testing solution. X-ray fluorescence (XRF) spectroscopy was performed in order to investigate the chemical structure of the coatings. The obtained results were analyzed regarding the AF agent as well as the flow/stationary conditions, which have influenced certain results.
Materials and Methods
Naval steel plates with the dimensions 120 mm × 70 mm × 3 mm were pre-treated before coating by shot-blasting to cleanliness grade Sa 2.5 according to ISO 8501-1, and thus all rust and foreign matter were completely removed. Thereafter, the plates were coated with the AC coating, the tie coat, and the AF coating. The AC coating was applied in two layers with a DFT of 150 µm each, that is, 300 µm in total, whereas the tie coat was applied at 100 µm. Thus, a coating system which is typically applied on the hull surface was applied on the plates’ surfaces. Two different AF coatings were used, a red acrylate self-polishing coating based on copper (AF1) and a gray biocide-free coating based on miscellaneous components (AF2). All the coatings were applied and dried according to the paint manufacturer’s instructions. The main characteristics of the tested coatings are presented In Table I.
The dry film thickness (DFT) of the applied coating system was measured according to ISO 2808,25 using a non-destructive magnetic induction method with an Elcometer 456 instrument (Elcometer, Manchester, UK). Measurements of DFT were carried out at ten different locations per plate. Roughness parameters including arithmetical mean deviation (Ra), root mean square (Rq), the average distance between peaks (Rz) and maximum height (Rt) of the roughness profile were measured using a MedioTehna Roughness Tester ART 100, at ten different locations per plate. The coating adhesion was assessed according to ISO 462426 using an Elcometer 108 Hydraulic Adhesion Tester (Elcometer). The chemical composition of the applied coating system was investigated using an XRF Analyzer DELTA Innov-X (Olympus).
The corrosion performance of the coated plates was predicted by accelerated laboratory tests which included the salt spray and the humidity chamber tests. The salt chamber test was conducted for 1440 h in accordance with ISO 9227,27 in 5% neutral NaCl solution in an Ascott cabinet, model S450 (Ascott Analytical Equipment, Staffordshire, UK). Before salt spray testing, the painted panels were horizontally scribed through the paint film to expose the base metal (ISO 7253). The humidity test was performed for 720 h in accordance with ISO 6270-2.28 Two coated plates per coating were tested within the salt spray and humidity chamber tests. Plates were periodically inspected in accordance with ISO 462829 to evaluate the degradation of the coatings.
The corrosion and antifouling properties of the coatings in natural seawater were evaluated by a 60-day immersion test. The seawater was sampled from the Adriatic Sea in Pula, Croatia (salinity 38.08‰, pH = 8). The plates were immersed into two 10-l containers. Thus, four plates were immersed in the container with a small propeller operating at 2800 rpm (M plates) and the others were immersed in the container without a propeller (S plates), simulating the ship navigating and the time when the ship was in the harbor. This was done in order to investigate the influence of introduced turbulence on the performance of the AF coatings.
The stability/tendency to corrosion in the seawater was tested with OCP measurements, whereas the protective properties of the applied coatings were examined by EIS measurements using a VersaSTAT 3 Potentiostat/Galvanostat (AMETEK Scientific 131 Instruments, Princeton Applied Research, Berwyn, PA, USA). The measurements were performed for each plate during a period of 60 days in the seawater at room temperature (23 ± 2°C). EIS was performed at OCP with a 10-mV sinusoidal amplitude in the frequency range from 100 kHz to 0.1 Hz. The working electrode was the tested plate, while Hastelloy C276 was the reference electrode. The area of the working electrode was 32 cm2. EIS measurements were repeated twice for each plate. For interpretation of the obtained data, the ZSimpWin Version 3.2 software was used. The protective properties were evaluated according to ISO 4628.
The antifouling properties were evaluated by visual assessment of the fouled surface and testing of surface roughness after the immersion test. Additionally, antifouling properties were reviewed by a spectrophotometer (DR/2400; Hach, CO, USA) by measuring the optical density at the wavelength of 600 nm before and after 120-h immersion of samples in 5 g/L NaCl solution containing 5 g/L of Saccharomyces cerevisiae at 20°C.
Results and Discussion
Measurement of DFT and Roughness: Chemical Composition of Applied Coating Systems
The measurement of DFT revealed that the average DFT of the coating system coated with AF1 was 515–625 µm (samples 1–6), while the average DFT of coating system coated with AF2 was 480–560 µm (samples 7–12). The roughness measurement revealed that the average Ra, Rz, Rq, and Rt of the coating system coated with AF1 were around 0.49 µm, 2.69 µm, 0.63 µm, and 3.85 µm, respectively, while the average Ra, Rz, Rq, and Rt of the coating system coated with AF2 were around 0.53 µm, 3.03 µm, 0.68 µm, and 4.32 µm, respectively.
Using the DELTA Professional Olympus Innov-X instrument, it was determined that the plates coated with AF1 have the highest amount of copper, but they also have a share of iron and sulfur. On the other hand, plates coated with AF2 have the highest amount of silicon followed by titanium, aluminum, iron, zinc, etc. The XRF results are presented in Table II.
Chamber Testing
The plates tested in the humidity chamber (ISO 6270-2) for a period of 30 days (720 h) did not exhibit any rusting or blistering (ISO 4628).29 However, the plates tested in the salt spray chamber test (ISO 9227) for a period of 60 days (1440 h) exhibited rusting of the undercoated surface. At the end of the test, the corrosion around the scribe was measured at six points and then the averaged value was calculated. The rust creep for AF1 coating was equal to 1.75 mm for plate 1, and 1.13 mm for plate 4. The possible reason for this is because plate 1 had some lower DFT. The rust creep for the AF2 coating was equal to 1.60 mm for plate 9, and 1.93 mm for plate 10. According to ASTM D1654 – 05,30 all the tested plates obtained 8 out of 10 grades of corrosion.
After the chamber testing, the DFT of coating system was measured again. The average value of the DFT on AF1 was reduced after the chamber testing. The DFT reduction for the AF1 plates that were exposed in the salt spray chamber was 62 µm, while the DFT reduction for plates after humidity chamber testing was 34.95 µm. The DFT on AF2 remained the same.
Furthermore, the adhesion of the coating system was tested using the pull-off test. All coating systems showed poor adhesion according to Ref. 26. The average pull-off adhesion for AF1 after the salt chamber was 1.415 ± 0.113 MPa (± SD), whereas for AF2 it was equal to 1.04 ± 0.29 MPa. After the humidity chamber testing, the obtained pull-off adhesion for AF1 was 1.405 ± 0.099 MPa, whereas for AF2 it was 1.135 ± 0.485 MPa. Cohesion failure in the antifouling coating was noticed in all the samples.
Immersion Test
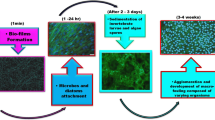
The plates which were immersed in seawater for 60 days (1440 h) experienced a significant appearance of biofilm (Fig. 1). Biofilm first appeared on the M plates (after 14 days on AF1 and 21 days on AF2) and later on the S plates (after 42 days). Also, a thicker biofilm was present on the M plates immersed in seawater with agitation. This can be attributed to the propeller operation, which brought a larger amount of air into the container. Therefore, the microorganisms maturated more quickly in the container with M plates. This was especially pronounced on the M plates protected with the biocide-free AF2 coating (7 M, 8 M) where microorganism colonization was noticed, whereas on the M plates protected with self-polishing AF1 coating (5 M, 6 M) only a relatively thick biofilm was determined. Both AF coatings experienced similar biofilm thickness after immersion in seawater without agitation (S plates). DFT was measured after the immersion test and no significant reduction in thickness was present. However, biofilm was present at all the plates.
After the immersion test, the surface of the tested plates was significantly rougher (Table III). As can be seen from Table III, a significantly rougher condition was present on the plates that were immersed in the seawater with an operating propeller (M plates), than on the plates that were immersed stationary (S plates). The roughness results after the immersion test correspond to the visual observation of fouling on the M samples compared to the S samples.
The antifouling capacity was reviewed using a spectrophotometer by immersion of the samples in NaCl solution containing Saccharomyces cerevisiae. The optical density at 600 nm of the testing solution was 2.732, whereas after 120-h immersion of the AF1 coating it was 0.921, and for AF2 it was 1.256. From the results obtained, it can be concluded that coating AF1 exhibits the stronger antibacterial activity.
The adhesion of the coating systems was tested using the pull-off test. All the coating systems showed poor adhesion according to Ref. 26. The average pull-off adhesion after the immersion test with agitation (M samples) for AF1 was 1.435 ± 0.245 MPa and for AF2 it was 0.845 ± 0.045 MPa, whereas the pull-off adhesion after immersion test without agitation (S samples) for AF1 was 1.315 ± 0.165 MPa and for AF2 it was 0.905 ± 0.095 MPa. Cohesion failure in the top coat, i.e., the antifouling coating was noticed in all the samples.
OCP and EIS
The results of OCP for the immersed plates after stabilization for 30 min in seawater are presented in Table IV. As said before, OCP was measured against the Hastelloy C276 and the obtained potential is given in volts.
As can be seen from Table IV, the OCP for all the coating systems varied with time. Thus, after 60 days of immersion, the OCP after stabilization in seawater was lower than before immersion for all coating systems, except 5 M and 5 S, where the OCP had grown. The significant variations in OCP can be attributed to the occurrence of biofilms, which influenced the OCP values.
The impedance data for the investigated coating systems were fitted by three equivalent electric circuits (Fig. 2).
The upper equivalent electric circuit shown in Fig. 2 was fitted to some impedance data for coating systems at the beginning of exposure to seawater. Thus, the Randles equivalent circuit was used for describing non-porous coating behavior.22 The middle equivalent electric circuit shown in Fig. 2 was fitted to the porous coating systems,20,22 without the presence of biofilms, while the lower equivalent electric circuit was fitted to the porous coating system with the presence of biofilms. These equivalent electric circuits consisted of the solution resistance, RS, the polarization resistance, RP, the coating capacitance, CC, the coating resistance, RC, the surface film capacitance,14CF, the surface film resistance,14RF, the double layer capacitance, Cdl, and the charge transfer resistance, Rct.
The results of the EIS analysis are given in Table V. The presented results are given for 4 days after the beginning of the immersion test and after the immersion test. It should be noted that within the first column the name of the plate is given, along with the time of the exposure in seawater. Thus, plates 6 S and 8 S were exposed 33 days, plates 5 S and 6 M for 42 days, while the other plates were exposed for 60 days. CPE is a constant phase element, which shows capacitive properties depending on the empirical constant, nC, which ranges from 0 to 1. For n = 0, CPE operates as a resistor, while for n = 1, CPE operates as a capacitor.22
As can be seen from Table V, after 4 days of immersion in the seawater, all the coating systems showed a porous behavior, except 5 S. The largest coating resistance was found for the AC–AF coating system, which had the self-polishing coating 5 M, while the lowest coating resistance was found for the AC–AF coating system which had FR coating 8 S. Coating performance is often judged using coating resistance.22 After longer immersion times, all the coating systems showed a porous behavior and a significant decrease in resistance. The highest coating resistance among the plates which were exposed for 60 days was found for the 5 M coating system with self-polishing AF and was equal to 1.302 103 Ω. In addition, the coating system 6 S showed higher coating resistance than the coating system 8 S after a 33-day immersion in seawater. The coating system with the self-polishing AF coating showed better corrosion protection than the FR coating. In addition, samples tested in stationary conditions (samples S) showed higher coating resistance compared to samples tested in agitation conditions (samples M). The coating system 7 S and 8 S tested in stationary conditions showed higher coating resistance than samples 7 M and 8 M in agitation, after the exposure of 60 days. In addition, a very important factor for corrosion protection is the surface condition and the occurrence of biofilms. Since all the plates had significantly rougher surfaces after the immersion test, the corrosion process was accelerated.31
EIS results for one representative of AF1 and AF2, both measured (Msd.) and fitted (Calc.) are presented in Bode and Nyquist diagrams (Fig. 3).
Conclusion
This study examined the protective properties of two different AC–AF coating systems, which differed in the AF coating. One was a self-polishing AF based on copper and the other was a new biocide-free fouling release AF based on miscellaneous components. The investigations carried out within this research showed good properties of the tested coating systems in the humid and warm environment, but also some negative properties in the simulated marine environment after salt spray testing and the immersion in seawater. The measurements of corrosion creep and pull-off adhesion after the salt spray chamber testing showed better protection behavior of the tested AF1 coating system compared to the AF2. The pull-off test on all the tested samples has shown that adhesion of the coatings after the exposure was poor, but this was noticeable only in the antifouling coat.
The reduction in DFT for AF1 was noticed after the chamber testing, especially after the salt-spray chamber test. This is possibly due to hydrolysis of the self-polishing AF binder in contact with water as well as due to erosion from the salt spray test.
The coating resistance for all the tested coating systems dropped significantly after the immersion test. Furthermore, the EIS study has shown that, for corrosion protection after the performed immersion test, the chemical composition of the coating system has more influence than its thickness, that is, the coating system coated with self-polishing AF showed better resistance than the coating system with a biocide-free FR coating for the same thickness after a 60-day immersion. Also, the occurrence of the biofilms on the plates caused the increase of the corrosion process, as the surface of the plates became significantly rougher. The occurrence of the biofilms was greater for the plates which were immersed with agitation. This can be attributed to the propeller operation since a larger amount of air was brought into the container. Thus, the microorganisms maturated more quickly in the container with M plates. Consequently, a larger drop of resistance was noticed for the plates which were immersed with agitation. Also, in seawater with agitation, the growth of the microorganisms was greater on the plates protected with biocide-free AF based on miscellaneous components than on the self-polishing AF coating based on copper. A higher antifouling capacity of the self-polishing AF coating was additionally confirmed by immersion of coatings in Saccharomyces cerevisiae solution.
References
P. Jurišić, J. Parunov, and Y. Garbatov, Brodogradnja 68, 15 (2017).
G.D. Bixler and B. Bhushan, Philos. Trans. R. Soc. A 370, 2381 (2012).
C.A. Damon, C.M. Gatley, J.J. Beres, J.A. Finlay, S.C. Franco, A.S. Clare, and M.R. Detty, Biofouling 32, 883 (2016).
J.S. Park and J.H. Lee, Biofouling 34, 98 (2018).
M. Menesses, J. Belden, N. Dickenson, and J. Bird, Biofouling 33, 703 (2017).
C. Ventura, A.J. Guerin, O. El-Zubir, A.J. Ruiz-Sanchez, L.I. Dixon, K.J. Reynolds, M.L. Dale, J. Ferguson, A. Houlton, B.R. Horrocks, and A.S. Clare, Biofouling 33, 892 (2017).
A. Mathiazhagan and R. Joseph, Int. J. Chem. Eng. 2, 225 (2011).
Z.P. Zhang, X.F. Song, L.Y. Cui, and Y.H. Qi, Coatings 8, 157 (2018).
M. Ba, Z. Zhang, and Y. Qi, Coatings 8, 153 (2018).
AMBIO, The Publishable Final Activity Report of the AMBIO Integrated Project (European Commission CORDIS Publications, 2010), https://cordis.europa.eu/publication/rcn/10830_en.html. Accessed 6 September 2018.
R.S. Peres, E. Armelin, J.A. Moreno-Martínez, C. Alemán, and C.A. Ferreira, Appl. Surf. Sci. 341, 75 (2015).
W. Cai, J. Wang, X. Quan, S. Zhao, and Z. Wang, Surf. Coat. Technol. 334, 7 (2018).
M. Hecker, M.S.H. Ting, and J. Malmström, Coatings 8, 55 (2018).
H. Li, C. Yang, E. Zhou, C. Yang, H. Feng, Z. Jiang, D. Xu, T. Gu, and K. Yang, J. Mater. Sci. Technol. 33, 1596 (2017).
S. Chen and D. Zhang, Corros. Sci. 136, 275 (2018).
M. Perez, M. Garcia, and G. Blustein, Mar. Environ. Res. 109, 177 (2015).
L.D. Chambers, J.A. Wharton, R.J.K. Wood, F.C. Walsh, and K.R. Stokes, Prog. Org. Coat. 77, 473 (2014).
M. Schönleber, D. Klotz, and E. Ivers-Tiffée, Electrochim. Acta 131, 20 (2014).
E.D. Kiosidou, A. Karantonis, and D.I. Pantelis, Chem. Eng. Trans. 41, 301 (2014).
N. Wang, H. Gao, J. Zhang, and P. Kang, Coatings 8, 179 (2018).
R. de Siqueira Melo, S.L.D.C. Brasil, L.J. de Carvalho, A.M. Limaverde Filho, and C. Pereira, Int. J. Electrochem. Sci. 11, 7750 (2016).
I. Stojanović, V. Šimunović, V. Alar, and F. Kapor, Coatings 8, 98 (2018).
S. Satheesh, M.A. Ba-akdah, and A.A. Al-Sofyani, Electron. J. Biotechnol. 21, 26 (2016).
L.D. Chambers, K.R. Stokes, F.C. Walsh, and R.J.K. Wood, Surf. Coat. Technol. 201, 3642 (2006).
ISO 2409 Paints and Varnishes—Cross-Cut Test; International Organization for Standardization: Geneva, Switzerland, 2013.
ISO 4624 Paints and Varnishes—Pull-off test for adhesion; International Organization for Standardization: Geneva, Switzerland, 2016.
ISO 9227 Corrosion Tests in Artificial Atmospheres—Salt Spray Tests; International Organization for Standardization: Geneva, Switzerland, 2017.
ISO 6270-2 Paints and Varnishes—Determination of Resistance to Humidity—Part 2: Condensation (In-Cabinet Exposure with Heated Water Reservoir); International Organization for Standardization: Geneva, Switzerland, 2017.
ISO 4628 Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance; International Organization for Standardization: Geneva, Switzerland, 2016.
ASTM D1654 – 05, Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments, ASTIM International.
P. Gümpel and A. Hörtnagl, Mater. Corros. 67, 607 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stojanović, I., Farkas, A., Alar, V. et al. Evaluation of the Corrosion Protection of Two Underwater Coating Systems in a Simulated Marine Environment. JOM 71, 4330–4338 (2019). https://doi.org/10.1007/s11837-019-03669-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03669-4