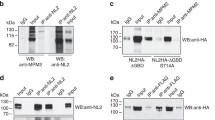
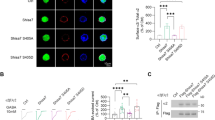
Abstract
Most fast synaptic inhibitions in the mammalian brain are mediated by GABAA receptors (GABAARs). An appropriate level of GABAAR expression at the cell surface is essential for neurodevelopment and the efficacy of GABAergic synaptic transmission. We previously reported that brefeldin A–inhibited GDP/GTP exchange factor 1 (BIG1), a binding partner of GABAARs, plays an important role in trafficking GABAARs to the cell surface. However, its regulatory mechanisms remain unknown. In the present study, we identified a new cellular protein, 14-3-3ζ, which can interact with the β subunit of GABAARs and BIG1 both in vitro and in vivo and colocalizes in the soma, dendrites, and axons of hippocampal neurons. Overexpression of 14-3-3ζ-WT increased the surface expression of BIG1 in dendrites and axons, as well as the binding of BIG1 with GABAAR. Depleted 14-3-3ζ with efficacious siRNA attenuated the interaction between BIG1 and GABAARs and resulted in significant decreases in the surface expression levels of BIG1 and GABAAR. GABAAR agonist treatment increased the expression levels of BIG1 and 14-3-3ζ on the surface, indicating that 14-3-3ζ is involved in regulating BIG1-mediated GABAAR surface expression. Depletion of BIG1 or 14-3-3ζ significantly decreased GABAAR expression at the cell surface and suppressed the GABA-gated influx of chloride ions. These data indicate that the combination of 14-3-3ζ and BIG1 is required for GABAAR membrane expression. Our results provide a potential promising therapeutic target for neurological disorders involving GABAergic synaptic transmission.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ, International Union of Pharmacology. XV (1998) Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50(2):291–313
Luscher B, Fuchs T, Kilpatrick CL (2011) GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70(3):385–409
Benarroch EE (2007) GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology 68(8):612–614
Kalueff AV, Nutt DJ (2007) Role of GABA in anxiety and depression. Depress Anxiety 24(7):495–517
Charych EI, Liu F, Moss SJ, Brandon NJ (2009) GABA(A) receptors and their associated proteins: implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology 57(5-6):481–495
Bravo-Hernandez M, Corleto JA, Barragan-Iglesias P, Gonzalez-Ramirez R, Pineda-Farias JB, Felix R, Calcutt NA, Delgado-Lezama R et al (2016) The alpha5 subunit containing GABAA receptors contribute to chronic pain. Pain 157(3):613–626
Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M et al (2008) Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451(7176):330–334
Kumar S, Fleming RL, Morrow AL (2004) Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther 101(3):211–226
Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW (1999) GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature 397(6714):69–72
Leil TA, Chen ZW, Chang CS, Olsen RW (2004) GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci 24(50):11429–11438
Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B (2004) The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci 24(26):5881–5891
Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B (2006) GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci 26(49):12758–12768
Gilbert SL, Zhang L, Forster ML, Anderson JR, Iwase T, Soliven B, Donahue LR, Sweet HO et al (2006) Trak1 mutation disrupts GABA(A) receptor homeostasis in hypertonic mice. Nat Genet 38(2):245–250
Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL (2004) The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem 90(1):173–189
Li C, Chen S, Yu Y, Zhou C, Wang Y, Le K, Li D, Shao W et al (2014) BIG1, a brefeldin A-inhibited guanine nucleotide-exchange factor, is required for GABA-gated Cl(-) influx through regulation of GABAA receptor trafficking. Mol Neurobiol 49(2):808–819
Liu L, Zhang S, Wang Y, Bao W, Zhou Y, Dang W, Wang X, Li H et al (2020) BIG1 controls macrophage pro-inflammatory responses through ARF3-mediated PI(4,5)P2 synthesis. Cell Death Dis 11(5):374
Lin S, Zhou C, Neufeld E, Wang YH, Xu SW, Lu L, Wang Y, Liu ZP et al (2013) BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein modulates ATP-binding cassette transporter A-1 trafficking and function. Arterioscler Thromb Vasc Biol 33(2):e31–e38
Sun M, Han X, Zhou D, Zhong J, Liu L, Wang Y, Ni J, Shen X et al (2021) BIG1 mediates sepsis-induced lung injury by modulating lipid raft-dependent macrophage inflammatory responses. Acta Biochim Biophys Sin Shanghai 53(8):1088–1097
Wright J, Kahn RA, Sztul E (2014) Regulating the large Sec7 ARF guanine nucleotide exchange factors: the when, where and how of activation. Cell Mol Life Sci 71(18):3419–3438
Zhou C, Li C, Li D, Wang Y, Shao W, You Y, Peng J, Zhang X et al (2013) BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein regulates neurite development via PI3K-AKT and ERK signaling pathways. Neuroscience 254:361–368
Teoh JJ, Iwano T, Kunii M, Atik N, Avriyanti E, Yoshimura SI, Moriwaki K, Harada A (2017) BIG1 is required for the survival of deep layer neurons, neuronal polarity, and the formation of axonal tracts between the thalamus and neocortex in developing brain. PLoS One 12(4):e0175888
Boston PF, Jackson P, Thompson RJ (1982) Human 14-3-3 protein: radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. J Neurochem 38(5):1475–1482
Berg D, Holzmann C, Riess O (2003) 14-3-3 proteins in the nervous system. Nat Rev Neurosci 4(9):752–762
Foote M, Zhou Y (2012) 14-3-3 proteins in neurological disorders. Int J Biochem Mol Biol 3(2):152–164
Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, Inestrosa NC (2010) Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci 30(25):8411–8420
Czondor K, Ellwanger K, Fuchs YF, Lutz S, Gulyas M, Mansuy IM, Hausser A, Pfizenmaier K et al (2009) Protein kinase D controls the integrity of Golgi apparatus and the maintenance of dendritic arborization in hippocampal neurons. Mol Biol Cell 20(7):2108–2120
Shen X, Meza-Carmen V, Puxeddu E, Wang G, Moss J, Vaughan M (2008) Interaction of brefeldin A-inhibited guanine nucleotide-exchange protein (BIG) 1 and kinesin motor protein KIF21A. Proc Natl Acad Sci U S A 105(48):18788–18793
Shen X, Hong MS, Moss J, Vaughan M (2007) BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein, is required for correct glycosylation and function of integrin beta1. Proc Natl Acad Sci U S A 104(4):1230–1235
Hoffman JF, Geibel JP (2005) Fluorescent imaging of Cl- in Amphiuma red blood cells: how the nuclear exclusion of Cl- affects the plasma membrane potential. Proc Natl Acad Sci U S A 102(3):921–926
Eshaq RS, Stahl LD, Stone R 2nd, Smith SS, Robinson LC, Leidenheimer NJ (2010) GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABAA receptors. Brain Res 1346:1–13
Porcher C, Hatchett C, Longbottom RE, McAinch K, Sihra TS, Moss SJ, Thomson AM, Jovanovic JN (2011) Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J Biol Chem 286(24):21667–21677
Jacob TC, Moss SJ, Jurd R (2008) GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 9(5):331–343
Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M (2002) Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: comparison with PRIP-1. Life Sci 72(4-5):443–453
Fujii M, Kanematsu T, Ishibashi H, Fukami K, Takenawa T, Nakayama KI, Moss SJ, Nabekura J, Hirata M (2010) Phospholipase C-related but catalytically inactive protein is required for insulin-induced cell surface expression of gamma-aminobutyric acid type A receptors. J Biol Chem 285(7):4837–4846
Moss J, Vaughan M (1995) Structure and function of ARF proteins: activators of cholera toxin and critical components of intracellular vesicular transport processes. J Biol Chem 270(21):12327–12330
Li H, Adamik R, Pacheco-Rodriguez G, Moss J, Vaughan M (2003) Protein kinase A-anchoring (AKAP) domains in brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2). Proc Natl Acad Sci U S A 100(4):1627–1632
Kuroda F, Moss J, Vaughan M (2007) Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma. Proc Natl Acad Sci U S A 104(9):3201–3206
Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN et al (2005) Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A 102(41):14871–14876
McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG (1998) Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci 1(1):23–28
Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ (2000) GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem 275(49):38856–38862
McDonald BJ, Moss SJ (1994) Differential phosphorylation of intracellular domains of gamma-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J Biol Chem 269(27):18111–18117
Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A et al (2006) Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3(1):47–58
Cheah PS, Ramshaw HS, Thomas PQ, Toyo-Oka K, Xu X, Martin S, Coyle P, Guthridge MA et al (2012) Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3zeta deficiency. Mol Psychiatry 17(4):451–466
Ramshaw H, Xu X, Jaehne EJ, McCarthy P, Greenberg Z, Saleh E, McClure B, Woodcock J et al (2013) Locomotor hyperactivity in 14-3-3zeta KO mice is associated with dopamine transporter dysfunction. Transl Psychiatry 3:e327
Workman ER, Haddick PC, Bush K, Dilly GA, Niere F, Zemelman BV, Raab-Graham KF (2015) Rapid antidepressants stimulate the decoupling of GABA(B) receptors from GIRK/Kir3 channels through increased protein stability of 14-3-3eta. Mol Psychiatry 20(3):298–310
Qian Z, Micorescu M, Yakhnitsa V, Barmack NH (2012) Climbing fiber activity reduces 14-3-3-theta regulated GABA(A) receptor phosphorylation in cerebellar Purkinje cells. Neuroscience 201:34–45
Shikano S, Coblitz B, Wu M, Li M (2006) 14-3-3 proteins: regulation of endoplasmic reticulum localization and surface expression of membrane proteins. Trends Cell Biol 16(7):370–375
Nakamura T, Sakaue F, Nasu-Nishimura Y, Takeda Y, Matsuura K, Akiyama T (2018) The autism-related protein PX-RICS mediates GABAergic synaptic plasticity in hippocampal neurons and emotional learning in mice. EBioMedicine 34:189–200
Nakamura T, Hayashi T, Mimori-Kiyosue Y, Sakaue F, Matsuura K, Iemura S, Natsume T, Akiyama T (2010) The PX-RICS-14-3-3zeta/theta complex couples N-cadherin-beta-catenin with dynein-dynactin to mediate its export from the endoplasmic reticulum. J Biol Chem 285(21):16145–16154
Krapf R, Berry CA, Verkman AS (1988) Estimation of intracellular chloride activity in isolated perfused rabbit proximal convoluted tubules using a fluorescent indicator. Biophys J 53(6):955–962
Kovalchuk Y, Garaschuk O (2012) Two-photon chloride imaging using MQAE in vitro and in vivo. Cold Spring Harb Protoc 2012(7):778–785
Acknowledgments
This work was supported by the "technology innovation 2030-major projects" on brain science and brain-like computing of the Ministry of Science and Technology of China (2021ZD0202603), the Guangdong Basic and Applied Basic Research Foundation (2022A1515012036), and the National Natural Science Foundation of China (31500841).
Funding
This work was supported by the “technology innovation 2030-major projects” on brain science and brain-like computing of the Ministry of Science and Technology of China (No. 2021ZD0202603), and the National Natural Science Foundation of China (grant number 31500841).
Author information
Authors and Affiliations
Contributions
Cuixian Li, Chun Zhou, and Jie Tang contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Cuixian Li and Jie Tang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at Southern Medical University. Principles established by the Committee of Southern Medical University on Animal Care were followed at all times.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Research involving Human Participants and/or Animals
No human subject was involved in this study. Procedures involving animal subjects were approved by the Institutional Animal Care and Use Committee (IACUC) at Southern Medical University.
Informed Consent
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Fig. S1.
BIG1 immunoprecipitation (IP) and Coomassie blue staining. Products of IP with control IgG or anti-BIG1 antibodies from 1 mg of fresh rat hippocampus homogenate were separated by 10% SDS–PAGE gel and stained with Coomassie R250 blue. After destaining, the gel was divided into four pieces for mass spectrometry analysis according to the centers of molecular weight marker bands from top to bottom (>170, ~55, ~50, 36-26 kDa). (PNG 181 kb)
Table S1.
Proteins from 36-26 kDa gel segments identified by more than two peptides in LC-MS/MS analyses. Proteins in gel block (36-26 kDa) were subjected to in-gel trypsin digestion and analyzed by LC–MS/MS. Normalized to the IgG pull down results, proteins pulled down by BIG1 with more than two unique peptides that represent the same target protein were shown. (DOCX 15 kb)
The blank control was analyzed with the vehicle (Krebs-HEPES) in SH-SY5Y cells labeled with the Cl– sensitive dye MQAE. Live cell images were taken every 5 seconds. (AVI 1166 kb)
Cl– influx stimulated by GABA (100 mM) in SHSY-5Y cells transfected with negative control siRNA (NC) was analyzed by a confocal microscope (Zeiss 710). Live cell images were taken every 5 seconds. (AVI 1278 kb)
Cl– influx stimulated by GABA (100 mM) in SHSY-5Y cells transfected with BIG1 siRNA (G05) was analyzed by a confocal microscope (Zeiss 710). Live cell images were taken every 5 seconds. (AVI 2598 kb)
Cl– influx stimulated by GABA (100 mM) in SHSY-5Y cells transfected with 14-3-3ζ siRNA (F06) was analyzed by a confocal microscope (Zeiss 710). Live cell images were taken every 5 seconds. (AVI 2823 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Huang, S., Peng, J. et al. 14-3-3ζ Mediates GABAAR Activation by Interacting with BIG1. Mol Neurobiol 60, 1721–1732 (2023). https://doi.org/10.1007/s12035-022-03172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03172-z