Abstract
To assess the effects of cutting phenology on early growth performance of three willow clones grown under different weed treatments and planting dates, freshly harvested (non-dormant) and cold-stored (dormant) cuttings from willow clone Tora, Jorr, and Olof were planted in bucket experiment outdoors in central Sweden on five planting dates (May–June 2013) with or without a model weed (spring barley). Non-dormant cuttings sprouted faster than dormant cuttings when planted early in the season. For cuttings planted later in the season, bud sprouting was affected only by willow clone. Aboveground biomass production was affected by cutting phenology, planting date, clone, and weed treatment. When planted on May 3 and May 10, biomass produced from non-dormant and dormant cuttings did not differ, while willows grown from dormant cuttings produced 59% more aboveground biomass than willows grown from non-dormant cuttings when planted on May 24–June 16. Tora produced on average 12% more biomass than Jorr and Olof, and weed competition reduced aboveground biomass production on average with 36%. The ability of willow to suppress weeds (WSA) was 26 (non-dormant cuttings) and 12% (dormant cuttings) higher for willows planted on May 3 compared with WSA of willows grown from cuttings planted later in the season. The ability to tolerate competition from weeds (WT) was 51 and 52% lower for willows grown from non-dormant and dormant cuttings planted late in the season compared with WT of willows planted earlier in the season. We conclude that planting with long-term cold storage of willow cuttings can be replaced with planting freshly harvested cuttings when planting is performed in early season, and that weed competition strongly reduces biomass production. Weed control during the establishment phase is crucial in order to maximize willow biomass production.
Similar content being viewed by others
Introduction
Improving the profitability of biomass production in willow short rotation coppice (SRC) is important for further implementation of this cropping system [1,2,3], and prospects for cost reduction are good for major cost components such as establishment and harvest [4]. In Sweden, willow SRC propagation units are routinely produced from dormant willow rods [5] which are harvested after growth cessation (i.e., early winter) and require storage in sub-zero temperatures, i.e., approximately − 4 °C [6] in order to retain vigor and vitality until planting. As cold storage is logistically demanding and adds to the costs, savings in planting material cold storage are desirable [7]. In general, the costs of willow SRC establishment are divided into material and field operation costs, which are approximately 80 and 20%, respectively. More than a half of the costs of material incur from purchase of planting material [8]. Moreover, as willow cuttings are nowadays commonly produced from dormant shoots, approximately 3–5% of the total cost of the planting material is attributed solely to cold storage (Lena Åsheim, Salixenergi Europa AB, Sweden, personal communication).
Grown from non-dormant propagation units, willows may survive, establish, and produce biomass, although these parameters may vary between different willow species/clones and abiotic conditions [7, 9, 10]. However, comparisons of the performance of non-dormant and dormant cuttings under conditions relevant for commercial willow SRC, such as different planting dates and weed pressure, are thus far unavailable.
Growth performance from cuttings is expected to depend on the available amount and activity of carbohydrates [11] and hormones [12] at planting and may also be dependent on willow clone [13, 14]. Cuttings that are non-dormant at planting presumably will establish faster than dormant cuttings. Faster establishment may in turn contribute to a higher competitive ability of willows under weed pressure, which is considered as a main determinant of willow SRC biomass production [15]. However, non-dormant cuttings that are harvested (and planted) later in the season may have depleted a part of their carbohydrate reserves and thereby have, in comparison to dormant cuttings planted later in the season, less reserves to grow and compete with weeds.
The aim of this study was to quantify early growth performance of willow as affected by cutting phenology. However, early growth parameters have been shown to be affected by interplay between different factors such as cutting phenology, planting date, and willow clone [9, 16]. Furthermore, weeding regime has been found to be an important predictor of early willow growth [15, 17]. With this in mind, we compared bud burst phenology, aboveground biomass production and the ability of willow to suppress weeds (WSA, weed suppressive ability of willow), and willow ability to tolerate competition from weeds (WT, weed tolerance of willow) of non-dormant and dormant cuttings from three willow clones planted at five planting dates with two levels of weed pressure.
We hypothesize that: (1) phenological development, in terms of bud burst, (a) will be faster for non-dormant cuttings compared with dormant ones at early planting dates, and (b) will be clone-dependent; (2) subsequent performance (aboveground biomass increment) of non-dormant and dormant cuttings will be dependent on cutting type, willow clone, planting date, and weed treatment; and (3) the differences in willow growth performance are in their turn reflected in willow WSA and WT.
Materials and Methods
Experimental Design
A bucket experiment was conducted outdoors in a netting enclosure at Ultuna near Uppsala, Sweden (59° 48′ N, 17° 39′ E) from May to September 2013. The buckets, with a volume of 12 l and a surface area of 0.064 m2, were filled with substrate, consisting of 85% moderately decomposed peat, 15% sand, total N content of 0.057 kg m−3, and NPK proportion of 2:1:2 (Hasselfors Garden AB, Sweden) and irrigated just before planting.
Overall, the experiment accommodated 240 buckets, containing willow cutting type (two levels; non-dormant and dormant), weed treatment (two levels; with and without weeds), willow clone (three levels; Tora, Jorr, and Olof), and planting date (five levels; May 3, 10, 24, and June 6, 16), all planted in four replicates. In order to avoid effects of competition for light between treatments, buckets were moved randomly within each planting date level during the first 5 weeks. To avoid damages of tall and branched shoots, random moving was omitted during weeks 6 to 8.
Cutting Preparation and Planting
Three willow clones commercially available and tested in numerous experiments in Sweden were used in the study: Tora (Salix schwerinii × Salix viminalis), Jorr (S. viminalis), and Olof (S. viminalis × (S. viminalis × S. schwerinii) [18]. For each of the willow clones, 80 1-year-old dormant shoots (each approximately 160-cm long and diameter approximately 1.0 cm at the shoot base) were tagged in a willow nursery at Ultuna, Uppsala on March 15, 2013. Per clone, 40 randomly chosen shoots were harvested, wrapped in polyethylene bags, and cold-stored (approximately − 4 °C). The shoots which remained in the field were randomly harvested on May 3, 10, and 24 and on June 6 and 16, 2013 in five batches of eight shoots per clone and planting date.
From both the basal and apical parts of all shoots, a 40-cm long part was removed to diminish the effect of dehydration, fungal/bacterial infections, and/or storage-caused damages. The remaining shoot parts were cut manually into four cuttings with a length of 20.0 ± 0.2 cm and diameter ranged from 0.8 (apical part of the shoot) to 1.9 cm (basal part of the shoot). Four willow cuttings were planted per bucket (giving a nominal planting density of about 65 cuttings m−2) by gently pressing them into the substrate while leaving approximately 2.0 cm of the cutting above the substrate surface.
In the weed treatment, a model weed, spring barley (Hordeum vulgare L. var. Waldemar, Svalöf Weibull AB, Malmö, Sweden), was sown 5 days after willow planting in order to ensure that willow sprouted in weed-free conditions. Sowing depth was approximately 2.0 cm and sowing density was 25 seeds bucket−1 (giving a nominal planting density of about 400 plants m−2). Spring barley was used as model weed due to its strong competitiveness and resemblance to monocotyledonous grassy weeds [19].
Irrigation was performed daily to eliminate competition for water. To avoid competition for nutrients, plants were fertilized 5 weeks after planting with a dose of 80, 16, and 70 kg N, P, and K ha−1 in a liquid form (Blomstra, WALLCO VÄXTNÄRING 51 + 10 + 43 + MIKRO, Cederroth International AB, Upplands Väsby, Sweden). Weeds occurring from the soil seed bank were manually removed. Monthly mean temperature during May–September 2013 ranged from 13.6 to 11.8 °C. Corresponding values for monthly precipitation and monthly radiation ranged from 14.6 to 52.8 mm and from 595.86 to 311.63 MJ m2, respectively [20].
Measurements
A day before each of the five planting dates, eight willow shoots per clone were taken out from cold store, and eight shoots per clone were freshly harvested from the willow nursery. On each shoot, bud burst developmental stage by using a five-stage scale [21] (Fig. 1) was assessed along the stem (S) between 40 and 120 cm above the shoot base and in the distal part of the shoot (D) about 150 cm above the shoot base.
The scale of bud burst developmental stages of willow shoots. Lower case letters represent stages of bud burst development cited from [21]. a Stage 1—no sign of bud swelling, the tip of the bud is tightly pressed to the shoot. b Stage 2—the tip of the bud starts to bend from the stem, bud scales are starting to open and the length of the shoot tip is 1–4 mm. c Stage 3—the shoot tip is 5 mm or longer, protruding leaves are put together. d Stage 4—new leaves start to bend from each other. e Stage 5—one or more new leaves are perpendicular to the shoot axis
For each willow cutting, bud burst phenology was recorded daily for the most developed bud per cutting [21].
Destructive harvest for each planting date was performed about 60 days after willow planting and coincided with a stage of 60–66 expanded leaves of willow clone Tora grown from dormant cuttings without weeds. Willow shoots (leaves and stems) were dried at 90 °C for 24 h; dry weight of each individual shoot was assessed and averaged per bucket. All barley shoots (stems, leaves, and spikes) were dried at 90 °C for 24 h, and dry weight was assessed per bucket.
Data Handling and Statistics
As there were differences in time span from planting to harvest and in temperature to which the experimental units planted on different dates were exposed, growing-degree day (GDD, °C) was calculated following McMaster and Wilhelm [22]:
where T max and T min represent the daily maximum and minimum air temperatures, respectively, and T base is the basal temperature (5 °C) below which willow is assumed to stop growing [23]. Calculations used measured T max and T min values, and Eq. (1) was used without any modifications [22]. Climatic records of air temperatures from the SLU meteorological station at Ultuna were used [20].
Daily GDD values were summed [24] over the period from planting to full sprouting (average bud burst phenology score = 5) giving bud burst cumulative growing-degree days (BCGDD, °C). In order to calculate increment of aboveground biomass production per GDD unit, the values of aboveground biomass were divided by the sum of daily GDD from willow planting to harvest.
Since willows and weeds for each planting date grew under the same time span and temperature, biomass production of willow and weeds used in the calculations of the WSA and WT remained uncorrected for GDD.
The WSA was calculated according to Nelson et al. [25]:
where b w denotes the total aboveground weed biomass and b t the total aboveground biomass (willow + weed) per experimental unit. The WT was calculated according to Szumigalski and Van Acker [26]:
where Cb w denotes the aboveground willow biomass in the presence of weeds and Cb wf the aboveground willow biomass when grown without weeds.
Willow growth parameters were modeled using PROC MIXED procedure in SAS [27] using restricted maximum likelihood (REML) method with Kenward-Roger denominator degrees of freedom adjustment [28] and with fixed effects of cutting type (two levels), willow clone (three levels), and planting date (five levels) (analyses of BCGDD); cutting type, weed treatment (two levels), willow clone, and planting date (analyses of willow aboveground biomass increment per GDD unit); and cutting type, planting date, and willow clone (analyses of WSA and WT). In the analyses of BCGDD, weed treatment was excluded as no competition from the weeds was assumed during the willow bud burst stage. The bucket (experimental unit) was a random variable in the model. The analyses (mixed-design ANOVA, post hoc comparisons of the means) were run on untransformed (originally measured) and transformed (log-transformed) datasets, and as they presented similar outcome (distribution of residuals), analyses on untransformed datasets were chosen. In all PROC MIXED analyses, post hoc multiple comparisons of the means were performed with Fisher’s least significant difference test at confidence level of 95%. Two-, three-, and four-way interactions between fixed effects were tested in the analyses.
Results
Bud Burst Phenology
The development of the bud burst of distal part of the shoots was more advanced compared with the middle part of the stem, and more advanced for non-dormant compared with dormant shoot material (Table 1).
Bud burst of cuttings expressed as BCGDD was significantly affected by cutting type (P = 0.0007), planting date (P < 0.0001), willow clone (P < 0.0001), by the two-way interactions (P < 0.0132), and by the three-way interaction (P = 0.0009) between these factors. The impact of experimental factors on BCGDD varied during the different planting dates (Table 2). The BCGDD was approximately 14 and 8% lower for non-dormant than dormant cuttings planted on May 3 and May 10, respectively (Table 2), but did not differ significantly between cutting types from May 24 to June 16. For willows planted on May 10–June 16, BCGDD was significantly affected by willow clone (Table 2). Willow clones Tora and Jorr developed faster than Olof on May 10 (non-dormant and dormant cuttings) and May 24 (non-dormant cuttings). Tora developed faster than Jorr and Olof on May 24 (dormant cuttings) and June 6 (dormant cuttings). Willow clone Olof developed faster than Jorr and Tora on June 6 (non-dormant cuttings), whereas development of willow clones on June 16 was Tora > Jorr > Olof (dormant cuttings) (Table 3).
Willow Aboveground Biomass Production
Willow aboveground biomass production per GDD unit was significantly affected by cutting type, weed treatment, planting date, willow clone (P < 0.0001 for all of them) and by some of their two-way interactions (except weed treatment × cutting type, planting date × willow clone, P > 0.2038). Neither three-way interactions (P > 0.1139) nor four-way interaction (P = 0.8397) significantly affected willow aboveground biomass production per GDD unit.
Overall, aboveground biomass production per GDD unit was significantly affected by cutting type but only for willows planted on May 24–June 16 (Table 4). Weed treatment had a significant effect on aboveground biomass production per GDD unit at all planting dates (P < 0.0001) (Table 4). Willow clone significantly affected biomass production per GDD unit at all planting dates except for May 3 (Table 4).
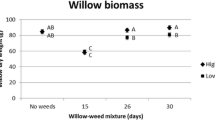
When investigated for separate cutting types, weed treatments, and willow clones (Table 5), aboveground biomass production per GDD unit was significantly reduced (P < 0.0006) for willows grown with weeds compared with willows grown without weeds. Depending on planting date: (i) biomass production per GDD unit was 34–84% lower when non-dormant cuttings grew with weeds, and (ii) biomass production per GDD unit was 52–72% lower when dormant cuttings grew with weeds (Fig. 2). When willows grew without weeds, no differences in biomass production per GDD unit between non-dormant and dormant cuttings were observed between planting dates May 3–June 6. On June 16, non-dormant cuttings produced significantly less (P = 0.0130) biomass per GDD unit than dormant cuttings (Table 5, Fig. 2). However, when willows grew with weeds, biomass production per GDD unit from non-dormant and dormant cuttings did not differ on May 3 and May 10 (P > 0.05). Non-dormant cuttings planted on May 24–June 16 produced significantly less biomass per GDD unit compared with dormant cuttings (P < 0.0130) (Fig. 2).
Aboveground biomass production per GDD unit (g GDD°C−1) from non-dormant and dormant willow cuttings grown with and without weeds planted at five different dates. Bars represent mean values with standard error of the means (±SE). Statistical significance (P < 0.05) within each of the five planting dates between weed treatments within cutting type or between cutting types within weed treatment is indicated by lower case and upper case letters, respectively
In several of the treatment combinations (planting date, cutting type, and weed treatment), the willow biomass produced per GDD unit (g GDD °C−1) differed significantly between willow clones (Table 5). Willow clone Olof grown from both non-dormant and dormant cuttings was frequently found to have the lowest biomass production per GDD unit (Table 5).
Willow WSA and WT
Willow WSA was significantly affected by cutting type, planting date, willow clone (P < 0.0001), by the two-way interactions (P ranged between < 0.0001 and 0.0262), but not by the three-way interaction (P = 0.1566) between these factors. The differences in WSA between different cutting types were insignificant on planting dates May 3 and May 10, but on May 24, June 6, and June 16, all willow clones grown from non-dormant cuttings had significantly lower (approximately 60% lower) WSA than willows grown from dormant cuttings (P = 0.0050, P = 0.0005, P = 0.0004 for willows planted on May 24, June 6, and June 16, respectively) (Fig. 3). Statistically significant differences in the WSA existed between willow clones but only on May 24 to June 16 (within cutting type, P ranged between < 0.0001 and 0.0102), and Tora and Olof had the highest and the lower WSA, respectively (Fig. 3).
Weed suppressive ability of willow (WSA, i.e., the ability of willow to suppress weeds). Bars represent treatment means with standard error of the means (±SE). Statistically significant differences in WSA within each of the five planting dates between non-dormant and dormant cuttings within and between willow clones are presented in upper right corner of each graph. Statistical significant differences (P < 0.05) in WSA within each of the five planting dates between non-dormant and dormant cuttings within individual willow clone or between willow clones irrespectively from cutting type are indicated by lower case and upper case letters, respectively. Note the difference in scales for plantings 1–4 and 5
Willow WT was significantly affected by cutting type (P = 0.0006), planting date (P = 0.0063), willow clone (P < 0.0001), by the two-way interaction cutting type × planting date (P = 0.0006), but not by the three-way interaction (P = 0.9680) between these factors. The differences in WT between different cutting types were insignificant on planting dates: May 3 and May 10, but on May 24 to June 16, all willow clones grown from non-dormant cuttings had significantly lower (approximately 52% lower) WT than willows grown from dormant cuttings (P < 0.0001, P = 0.0094, P = 0.0365 for willows planted on May 24, June 6, and June 16, respectively) (Fig. 4). Statistically significant differences in WT existed between willow clones but only during planting dates May 3 and June 6 (within cutting type, P = 0.0116 and P = 0.0428, respectively), and the highest WT was recorded for willow clone Tora (Fig. 4).
Weed tolerance of willow (WT, i.e., the ability of willow to tolerate competition from a model weed). Bars are treatment means with standard error of the means (±SE). Statistically significant differences in WT within each of the five planting dates between non-dormant and dormant cuttings within and between willow clones are presented in upper right corner of each graph. Statistical significant differences (P < 0.05) in WT within each of the five planting dates between non-dormant and dormant cuttings within individual willow clone or between willow clones irrespectively from cutting type are indicated by lower case and upper case letters, respectively
Discussion
Our study is the first report comparing early growth performance of non-dormant versus dormant willow cuttings in terms of bud burst, aboveground biomass production, and ability to suppress weeds and tolerate weeds.
The rationale behind the first hypothesis presented in the study was that the non-dormant cuttings mobilize their carbohydrate reserves, which will be more depleted in non-dormant than dormant cuttings the later the planting date becomes, and that BCGDD is genetically determined and differs between willow clones [29, 30]. The first hypothesis was partly supported by our results as BCGDD was significantly affected by willow clone during mid- and late planting dates (i.e., May 10 and 24, June 16). Frequently, the willow clone Olof required more BCGDD to full sprouting than other two willow clones which was also reported by Verwijst et al. [21]. The interaction between cutting type and willow clone increased in significance as a factor affecting BCGDD toward later planting dates, and dormant cuttings presented clear differences in BCGDD (Tora < Jorr < Olof) at the latest planting date (i.e., June 16). Significant differences in BCGDD were observed between non-dormant and dormant cuttings only on early planting dates (i.e., May 3 and May 10), and non-dormant cuttings required less BCGDD (meaning sprouted earlier) to achieve complete sprouting (from no bud swell to development of leaves) compared with dormant cuttings.
The significant effect of cutting type on bud burst phenology in early season (Table 1) suggests that bud burst in our study was affected rather by the hormonal factors than determined by the size of the carbohydrate reserves, which presumably was comparable for non-dormant and dormant cuttings planted on May 3 and May 10. While we standardized for differences in temperature sums between planting dates, it should be noted that both carbohydrate reserves mobilization and hormonal pathways in bud burst phenology are sensitive to other environmental factors such as day length, light conditions, and seasonality of temperature [31,32,33,34].
The aboveground biomass production per GDD unit was affected by planting date and weed treatment providing support for our second hypothesis. However, as the impact of cutting type and willow clone became significant only under certain circumstances, the hypothesis was only partially supported.
For all planting dates, the impact of weed competition on aboveground biomass production per GDD unit was significant (Fig. 2), confirming the need for weeding during willow SRC establishment [15]. When grown without weeds, aboveground biomass production per GDD unit was equal for non-dormant and dormant cuttings during all but the last planting date (i.e., June 16), at which the dormant ones performed slightly better (Fig. 2). This is likely due to a resource depletion of the non-dormant cuttings in late season. In the presence of weeds, however, biomass production of non-dormant cuttings planted on May 24–June 16 was lower compared with dormant cuttings, showing the combined effect of competition from weeds and declining carbohydrate reserves of non-dormant cuttings. While the effect of weeds was significant during the entire planting season, factors such as cutting type and willow clone became significant only under certain conditions, showing that final aboveground biomass production is dependent on the interactions of all factors studied. This is in agreement with other studies which account for the simultaneous impact of several factors on biomass production of willow propagated either from non-dormant or dormant willow cuttings. For example, Teodorescu et al. [9] reported that non-dormant willow shoots planted as green structures in urban environment in Canada varied in biomass production between willow clones and planting dates. Furthermore, biomass production from dormant cuttings of three willow clones (i.e., Tora, Doris, and Tordis) was approximately 55–89% higher when willows were planted in early May as compared with early July and varied between willow clones and geographical locations [16]. Clone- and site-dependent reduction of willow biomass with > 90% under weed competition in Swedish SRC willow propagated from dormant cuttings was reported by Albertsson et al. [15]. A study of Larsen et al. [35] reported that biomass production of willows propagated from dormant cuttings not only depended on willow clone but also on soil type, climate, and willow SRC management.
In our last hypothesis, we postulated that differences in willow growth performance are reflected in WSA and WT. Abundant irrigation and fertilization of plants in our experiment aimed to minimize competition for resources other than light, which is considered as the most essential factor at the phase of willow establishment and early growth. Thus, willow performance such as WSA and WT was in our study expressed predominantly in relation to competition for light.
Overall, WSA and WT were found to be very low, which supports the conclusion that limited light retards willow development and decreases competitive ability of willow during establishment phase [15]. This poor ability to suppress and to tolerate weeds does require efficient weed control during willow establishment [6, 36]. When planted later in the season, willows grown from non-dormant cuttings had a significantly lower WSA and WT than willows grown from dormant cuttings, whereas no significant differences in WSA and WT between willows grown from non-dormant and dormant cuttings were found early in the season (Figs. 3 and 4). This is likely due to the declining carbohydrate reserves of non-dormant cuttings later in the season. However, as WSA and WT are simple proportions between biomass of crop (in our case willow) and weed (in our case spring barley) grown in mixtures and in monocultures [25, 26], levels of WSA and WT also may be influenced by other factors than direct crop-weed interactions. Influence of organisms from other tropic levels, such as pests and predators which are specific to weeds or to willow clones, may have accounted for some of the variation encountered in this study. While the experiment was terminated 8 weeks after planting to avoid intraspecific competition between willow plants, WSA and WT also were likely to be affected by the initial planting density of both weeds and willow.
Conclusions
We conclude that when planted early in the growing season, non-dormant and dormant willow cuttings present similar aboveground biomass production and ability to suppress weeds and to tolerate weeds. Within a given weed treatment, and when planted later in the growing season, these parameters become dependent of clone for non-dormant and dormant willow cuttings. This implies that: (1) cold storage of cuttings is redundant when willow SRC is to be planted in early growing season, and (2) weeds have a strong and negative effect on willow growth during early establishment, whenever non-dormant or dormant cuttings are planted throughout the growing season. Consequently, weed control is crucial for a successful establishment and should be performed in order to maximize aboveground biomass production in willow SRC.
References
Buchholz T, Volk TA (2011) Improving the profitability of willow crops-identifying opportunities with a crop budget model. Bioenergy Res 4(2):85–95. https://doi.org/10.1007/s12155-010-9103-5
Toivonen RM, Tahvanainen LJ (1998) Profitability of willow cultivation for energy production in Finland. Biomass Bioenergy 15(1):27–37. https://doi.org/10.1016/s0961-9534(97)10056-3
Ericsson K, Rosenqvist H, Ganko E, Pisarek M, Nilsson L (2006) An agro-economic analysis of willow cultivation in Poland. Biomass Bioenergy 30(1):16–27. https://doi.org/10.1016/j.biombioe.2005.09.002
Rosenquist H, Berndes G, Borjesson P (2013) The prospects of cost reductions in willow production in Sweden. Biomass Bioenergy 48:139–147. https://doi.org/10.1016/j.biombioe.2012.11.013
Stantuf JA, van Oosten C (2014) Operational poplar and willow culture. In: Isebrands JG, Richardson J (eds) Poplars and willows: Trees for society and the environment. CAB International and FAO, Rome pp 200-247
Gustafsson J, Larsson S, Nordh NE (2007) Manual för salixodlare http://www.bioenergiportalen.se/attachments/42/406.pdf. Accessed 17 Jan 2017
McCracken AR, Moore JP, Walsh LRE, Lynch M (2010) Effect of planting vertical/horizontal willow (Salix spp.) cuttings on establishment and yield. Biomass Bioenergy 34(12):1764–1769. https://doi.org/10.1016/j.biombioe.2010.07.008
Caslin B, Larsson S, McCracken A (2010) Short rotation coppice willow—best practice guidelines. Teagasc, Crops Research Centre, Oak Park, Carlow, Ireland. http://www.seai/Renewables/Bioenergy/Willow_Best_Practice_Guide_2010.pdf. Accessed 17 Jan 2017
Teodorescu TI, Guidi W, Labrecque M (2011) The use of non-dormant rods as planting material: a new approach to establishing willow for environmental applications. Ecol Eng 37(9):1430–1433. https://doi.org/10.1016/j.ecoleng.2011.03.031
Pezeshki SR, Brown CE, Elcan JM, Douglas Shields F (2005) Responses of nondormant black willow (Salix nigra) cuttings to preplanting soaking and soil moisture. Restor Ecol 13(1):1–7. https://doi.org/10.1111/j.1526-100X.2005.00001.x
Carpenter LT, Pezeshki SR, Shields FD (2008) Responses of nonstructural carbohydrates to shoot removal and soil moisture treatments in Salix nigra. Trees 22(5):737–748. https://doi.org/10.1007/s00468-008-0234-7
Arora R, Rowland LJ, Tanino K (2003) Induction and release of bud dormancy in woody perennials: a science comes of age. Hortscience 38:911–921
Larsen SU, Jorgensen U, Laerke PE (2014) Willow yield is highly dependent on clone and site. Bioenergy Res 7(4):1280–1292. https://doi.org/10.1007/s12155-014-9463-3
Sevel L, Nord-Larsen T, Raulund-Rasmussen K (2012) Biomass production of four willow clones grown as short rotation coppice on two soil types in Denmark. Biomass Bioenergy 46:664–672. https://doi.org/10.1016/j.biombioe.2012.06.030
Albertsson J, Verwijst T, Hansson D, Bertholdsson NO, Ahman I (2014) Effects of competition between short-rotation willow and weeds on performance of different clones and associated weed flora during the first harvest cycle. Biomass Bioenergy 70:364–372. https://doi.org/10.1016/j.biombioe.2014.08.003
Nordh NE (2011) Planteringstidpunktens betydelse för etablering, tillväxt och överlevnad i Salix-odlingar. Slutrapport Projnr: H0640040
Edelfeldt S, Lundkvist A, Forkman J, Verwijst T (2016) Establishment and early growth of willow at different levels of weed competition and nitrogen fertilization. Bioenergy Res 9(3):763–772. https://doi.org/10.1007/s12155-016-9723-5
Caslin B, Finnan J, McCracken A (2012) Willow varietal identification guide. Teagasc, Oak Park, Carlow, Ireland
Watson PR, Derksen DA, Van Acker RC (2006) The ability of 29 barley cultivars to compete and withstand competition. Weed Sci 54(4):783–792. https://doi.org/10.1614/ws-05-020r3.1
Anonymous (2016) Ultuna climate station. SLU, Uppsala, Sweden. http://grodden.evp.slu.se/slu_klimat/slu_files/dygn.html. Accessed 20.06.2016
Verwijst T, Lundkvist A, Edelfeldt S, Forkman J, Nordh N-E (2012) Effects of clone and cutting traits on shoot emergence and early growth of willow. Biomass Bioenergy 37:257–264. https://doi.org/10.1016/j.biombioe.2011.12.004
McMaster GS, Wilhelm WW (1997) Growing degree-days: one equation, two interpretations. Agric For Meteorol 87(4):291–300. https://doi.org/10.1016/S0168-1923(97)00027-0
Sannervik AN, Eckersten H, Verwijst T, Kowalik P, Nordh N-E (2006) Simulation of willow productivity based on radiation use efficiency, shoot mortality and shoot age. Eur J Agron 24(2):156–164. https://doi.org/10.1016/j.eja.2005.07.007
Su LJ, Wang QJ, Bai YG (2013) An analysis of yearly trends in growing degree days and the relationship between growing degree day values and reference evapotranspiration in Turpan area, China. Theor Appl Climatol 113(3–4):711–724. https://doi.org/10.1007/s00704-012-0814-8
Nelson AG, Pswarayi A, Quideau S, Frick B, Spaner D (2012) Yield and weed suppression of crop mixtures in organic and conventional systems of the Western Canadian Prairie. Agron J 104(3):756–762. https://doi.org/10.2134/agronj2011.0374
Szumigalski A, Van Acker R (2005) Weed suppression and crop production in annual intercrops. Weed Sci 53(6):813–825. https://doi.org/10.1614/ws-05-014r.1
Cary NC (2011) SAS Institute Inc SAS/STAT® 9.3 User’s guide: the MIXED procedure (Chapter). Cary, SAS Institute, Inc
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Hallingback HR, Fogelqvist J, Powers SJ, Turrion-Gomez J, Rossiter R, Amey J, Martin T, Weih M, Gyllenstrand N, Karp A, Lagercrantz U, Hanley SJ, Berlin S, Ronnberg-Wastljung AC (2016) Association mapping in Salix viminalis L. (Salicaceae)—identification of candidate genes associated with growth and phenology. Glob Change Biol Bioenergy 8(3):670–685. https://doi.org/10.1111/gcbb.12280
Ronnberg-Wastljung AC (2001) Genetic structure of growth and phenological traits in Salix viminalis. Can J For Res-Revue Canadienne De Recherche Forestiere 31(2):276–282. https://doi.org/10.1139/cjfr-31-2-276
Rabot A, Henry C, Ben Baaziz K, Mortreau E, Azri W, Lothier J, Hamama L, Boummaza R, Leduc N, Pelleschi-Travier S, Le Gourrierec J, Sakr S (2012) Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol 53(6):1068–1082. https://doi.org/10.1093/pcp/pcs051
Reig C, Grillone N, Mesejo C, Martinez-Fuentes A, Agusti M (2016) Soil temperature regulates fruit color change in ‘Algerie’ loquat: nutritional and hormonal control. J Plant Growth Regul 35(4):1108–1117. https://doi.org/10.1007/s00344-016-9608-z
Basler D, Korner C (2014) Photoperiod and temperature responses of bud swelling and bud burst in four temperate forest tree species. Tree Physiol 34(4):377–388. https://doi.org/10.1093/treephys/tpu021
Murray MB, Cannell MGR, Smith RI (1989) Date of budburst of fifteen tree species in Britain following climatic warming. J Appl Ecol 26(2):693–700. https://doi.org/10.2307/2404093
Larsen SU, Jørgensen U, Lærke PE (2014) Willow yield is highly dependent on clone and site. BioEnergy Res 7(4):1280–1292. https://doi.org/10.1007/s12155-014-9463-3
Abrahamson LP, Volk TA, Smart LB, Cameron KD (2010) Shrub willow biomass producer’s handbook. State University of New York, Syracuse https://blogs.cornell.edu/willow/files/2014/10/ProducersHandbook-2enkbl7.pdf. Accessed 17 January 2017
Acknowledgments
The authors thank the Swedish Energy Agency (grant no. 35237-1) and the Swedish University of Agricultural Sciences (SLU) for funding this research, Nils-Erik Nordh, Richard Childs, Hanna Dahlén and Varwi Jacob Tavaziva for technical assistance, Johannes Forkman for valuable advice on statistical analyses, and two anonymous reviewers for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Welc, M., Lundkvist, A. & Verwijst, T. Effects of Cutting Phenology (Non-dormant Versus Dormant) on Early Growth Performance of Three Willow Clones Grown Under Different Weed Treatments and Planting Dates. Bioenerg. Res. 10, 1094–1104 (2017). https://doi.org/10.1007/s12155-017-9871-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-017-9871-2