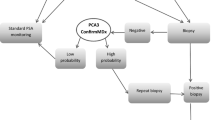
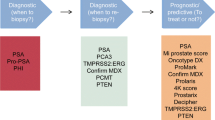
Abstract
Prostate cancer (PCa) screening and detection have changed dramatically since the introduction of serum prostate-specific antigen (PSA) testing. Despite the resulting improvement in early PCa detection and stage migration, in clinical practice the use of PSA testing may cause overdetection and ultimately overtreatment. As a consequence, novel biomarkers are needed to increase the specificity of PCa detection. The aim of this article is to present an overview of novel blood- and urine-based biomarkers that may optimize PCa detection, with improved identification of patients with significant PCa and avoidance of unnecessary prostate biopsies. A systematic and comprehensive PubMed search was performed using the MeSH search terms ‘prostate cancer’, ‘biomarker’, ‘marker’, and ‘detection’. Results were restricted to the English language. Several blood- and urine-based biomarkers have the potential to improve prediction of the presence and/or significance of PCa. Ideally, biomarkers should be used in combination within multivariate models, leading to superior accuracy for prediction of any PCa or clinically significant PCa, compared with the use of a single marker.
Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985–93.
Paquette EL, Sun L, Paquette LR, Connelly R, McLeod DG, Moul JW. Improved prostate cancer-specific survival and other disease parameters: impact of prostate-specific antigen testing. Urology. 2002;60:756–9.
Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5:e9584.
Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: a surveillance modeling approach. Med Decis Mak. 2008;28:323–31.
National Cancer Institute. Prostate cancer. 2012. http://www.cancer.gov/cancertopics/types/prostate. Accessed 7 Jul 2012.
Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8.
Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from US prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90.
Telesca D, Etzioni R, Gulati R. Estimating lead time and overdiagnosis associated with PSA screening from prostate cancer incidence trends. Biometrics. 2008;64:10–9.
Salagierski M, Schalken JA. Molecular diagnosis of prostate cancer: PCA3 and TMPRSS2:ERG gene fusion. J Urol. 2012;187:795–801.
Shariat SF, Semjonow A, Lilja H, Savage C, Vickers AJ, Bjartell A. Tumor markers in prostate cancer I: blood-based markers. Acta Oncol. 2011;50(Suppl 1):61–75.
Roobol MJ, Haese A, Bjartell A. Tumour markers in prostate cancer III: biomarkers in urine. Acta Oncol. 2011;50(Suppl 1):85–9.
Auprich M, Bjartell A, Chun FK, et al. Contemporary role of prostate cancer antigen 3 in the management of prostate cancer. Eur Urol. 2011;60:1045–54.
Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. 2003;95:634–5.
Vickers AJ, Kattan MW, Daniel S. Method for evaluating prediction models that apply the results of randomized trials to individual patients. Trials. 2007;8:14.
Shariat SF, Karam JA, Walz J, et al. Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood-based biomarkers. Clin Cancer Res. 2008;14:3785–91.
Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008;6:19.
Vickers AJ, Jang K, Sargent D, Lilja H, Kattan MW. Systematic review of statistical methods used in molecular marker studies in cancer. Cancer. 2008;112:1862–8.
Bensalah K, Montorsi F, Shariat SF. Challenges of cancer biomarker profiling. Eur Urol. 2007;52:1601–9.
Chun FK, Karakiewicz PI, Huland H, Graefen M. Role of nomograms for prostate cancer in 2007. World J Urol. 2007;25:131–42.
Bensalah K, Lotan Y, Karam JA, Shariat SF. New circulating biomarkers for prostate cancer. Prostate Cancer Prostatic Dis. 2008;11:112–20.
Verma M, Srivastava S. New cancer biomarkers deriving from NCI early detection research. Recent Results Cancer Res. 2003;163:72–84. discussion 264–6.
Winget MD, Baron JA, Spitz MR, et al. Development of common data elements: the experience of and recommendations from the early detection research network. Int J Med Inform. 2003;70:41–8.
Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–78.
Schroder FH, Carter HB, Wolters T, et al. Early detection of prostate cancer in 2007. Part 1: PSA and PSA kinetics. Eur Urol. 2008;53:468–77.
Shariat SF, Karam JA, Roehrborn CG. Blood biomarkers for prostate cancer detection and prognosis. Future Oncol. 2007;3:449–61.
Shariat SF, Karakiewicz PI, Margulis V, Kattan MW. Inventory of prostate cancer predictive tools. Curr Opin Urol. 2008;18:279–96.
Lilja H. Significance of different molecular forms of serum PSA. The free, noncomplexed form of PSA versus that complexed to alpha 1-antichymotrypsin. Urol Clin N Am. 1993;20:681–6.
Shariat SF, Abdel-Aziz KF, Roehrborn CG, Lotan Y. Pre-operative percent free PSA predicts clinical outcomes in patients treated with radical prostatectomy with total PSA levels below 10 ng/mL. Eur Urol. 2006;49:293–302.
Catalona WJ, Southwick PC, Slawin KM, et al. Comparison of percent free PSA, PSA density, and age-specific PSA cutoffs for prostate cancer detection and staging. Urology. 2000;56:255–60.
Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–7.
Woodrum DL, Brawer MK, Partin AW, Catalona WJ, Southwick PC. Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol. 1998;159:5–12.
Lee R, Localio AR, Armstrong K, Malkowicz SB, Schwartz JS. A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology. 2006;67:762–8.
Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination: enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452–5.
Rowe EW, Laniado ME, Walker MM, Patel A. Prostate cancer detection in men with a ‘normal’ total prostate-specific antigen (PSA) level using percentage free PSA: a prospective screening study. BJU Int. 2005;95:1249–52.
Pepe P, Panella P, Savoca F, et al. Prevalence and clinical significance of prostate cancer among 12,682 men with normal digital rectal examination, low PSA levels (< or = 4 ng/mL) and percent free PSA cutoff values of 15 and 20%. Urol Int. 2007;78:308–12.
Graefen M, Karakiewicz PI, Cagiannos I, et al. Percent free prostate specific antigen is not an independent predictor of organ confinement or prostate specific antigen recurrence in unscreened patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2002;167:1306–9.
Mikolajczyk SD, Marks LS, Partin AW, Rittenhouse HG. Free prostate-specific antigen in serum is becoming more complex. Urology. 2002;59:797–802.
Mikolajczyk SD, Marker KM, Millar LS, et al. A truncated precursor form of prostate-specific antigen is a more specific serum marker of prostate cancer. Cancer Res. 2001;61:6958–63.
Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–74.
Sokoll LJ, Sanda MG, Feng Z, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [−2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev. 2010;19:1193–200.
Jansen FH, van Schaik RH, Kurstjens J, et al. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol. 2010;57:921–7.
Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184–204.
Nam RK, Diamandis EP, Toi A, et al. Serum human glandular kallikrein-2 protease levels predict the presence of prostate cancer among men with elevated prostate-specific antigen. J Clin Oncol. 2000;18:1036–42.
Becker C, Piironen T, Pettersson K, Hugosson J, Lilja H. Clinical value of human glandular kallikrein 2 and free and total prostate-specific antigen in serum from a population of men with prostate-specific antigen levels 3.0 ng/mL or greater. Urology. 2000;55:694–9.
Becker C, Piironen T, Pettersson K, et al. Discrimination of men with prostate cancer from those with benign disease by measurements of human glandular kallikrein 2 (HK2) in serum. J Urol. 2000;163:311–6.
Vickers A, Cronin A, Roobol M, et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol. 2010;28:2493–8.
Haese A, Graefen M, Steuber T, et al. Human glandular kallikrein 2 levels in serum for discrimination of pathologically organ-confined from locally-advanced prostate cancer in total PSA-levels below 10 ng/mL. Prostate. 2001;49:101–9.
Kwiatkowski MK, Recker F, Piironen T, et al. In prostatism patients the ratio of human glandular kallikrein to free PSA improves the discrimination between prostate cancer and benign hyperplasia within the diagnostic “gray zone” of total PSA 4 to 10 ng/mL. Urology. 1998;52:360–5.
Recker F, Kwiatkowski MK, Piironen T, et al. The importance of human glandular kallikrein and its correlation with different prostate specific antigen serum forms in the detection of prostate carcinoma. Cancer. 1998;83:2540–7.
Kurek R, Nunez G, Tselis N, et al. Prognostic value of combined “triple”-reverse transcription-PCR analysis for prostate-specific antigen, human kallikrein 2, and prostate-specific membrane antigen mRNA in peripheral blood and lymph nodes of prostate cancer patients. Clin Cancer Res. 2004;10:5808–14.
Steuber T, Vickers A, Haese A, et al. Free PSA isoforms and intact and cleaved forms of urokinase plasminogen activator receptor in serum improve selection of patients for prostate cancer biopsy. Int J Cancer. 2007;120:1499–504.
Gupta A, Lotan Y, Ashfaq R, et al. Predictive value of the differential expression of the urokinase plasminogen activation axis in radical prostatectomy patients. Eur Urol. 2009;55:1124–33.
Hessels D, Klein Gunnewiek JM, van Oort I, et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol. 2003;44:8–15. discussion 15-6.
van Gils MP, Cornel EB, Hessels D, et al. Molecular PCA3 diagnostics on prostatic fluid. Prostate. 2007;67:881–7.
Deras IL, Aubin SM, Blase A, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol. 2008;179:1587–92.
Groskopf J, Aubin SM, Deras IL, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089–95.
Haese A, de la Taille A, van Poppel H, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol. 2008;54:1081–8.
Marks LS, Fradet Y, Deras IL, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69:532–5.
Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/mL or lower. JAMA. 2005;294:66–70.
US Food and Drug Administration. P100033: Progensa® PCA3 assay. 2012. http://www.accessdata.fda.gov/cdrh_docs/pdf10/p100033a.pdf. Accessed 15 Apr 2012.
Auprich M, Haese A, Walz J, et al. External validation of urinary PCA3-based nomograms to individually predict prostate biopsy outcome. Eur Urol. 2010;58:727–32.
Chun FK, de la Taille A, van Poppel H, et al. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol. 2009;56:659–67.
Hansen J, Auprich M, Ahyai SA, et al. Initial prostate biopsy: development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol. Epub 2012 Jul 20.
Nakanishi H, Groskopf J, Fritsche HA, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179:1804–9. discussion 1809-10.
van Poppel H, Haese A, Graefen M, et al. The relationship between prostate cancer gene 3 (PCA3) and prostate cancer significance. BJU Int. 2012;109:360–6.
Whitman EJ, Groskopf J, Ali A, et al. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J Urol. 2008;180:1975–8. discussion 1978-9.
Hessels D, van Gils MP, van Hooij O, et al. Predictive value of PCA3 in urinary sediments in determining clinico-pathological characteristics of prostate cancer. Prostate. 2010;70:10–6.
Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–8.
Hessels D, Smit FP, Verhaegh GW, Witjes JA, Cornel EB, Schalken JA. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin Cancer Res. 2007;13:5103–8.
Tomlins SA, Aubin SM, Siddiqui J, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72.
Salami SS, Schmidt F, Laxman B, et al. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol. Epub 2011 May 19.
Rice KR, Chen Y, Ali A, et al. Evaluation of the ETS-related gene mRNA in urine for the detection of prostate cancer. Clin Cancer Res. 2010;16:1572–6.
Demichelis F, Fall K, Perner S, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–9.
Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–63.
Gopalan A, Leversha MA, Satagopan JM, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–6.
Baden J, Green G, Painter J, et al. Multicenter evaluation of an investigational prostate cancer methylation assay. J Urol. 2009;182:1186–93.
Henrique R, Jeronimo C, Hoque MO, et al. Frequent 14-3-3 sigma promoter methylation in benign and malignant prostate lesions. DNA Cell Biol. 2005;24:264–9.
Hoque MO, Topaloglu O, Begum S, et al. Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects. J Clin Oncol. 2005;23:6569–75.
Schostak M, Schwall GP, Poznanovic S, et al. Annexin A3 in urine: a highly specific noninvasive marker for prostate cancer early detection. J Urol. 2009;181:343–53.
de la Taille A, Irani J, Graefen M, et al. Clinical evaluation of the PCA3 assay in guiding initial biopsy decisions. J Urol. 2011;185:2119–25.
Shariat SF, Karam JA, Margulis V, Karakiewicz PI. New blood-based biomarkers for the diagnosis, staging and prognosis of prostate cancer. BJU Int. 2008;101:675–83.
Shariat SF, Karakiewicz PI. Perspectives on prostate cancer biomarkers. Eur Urol. 2008;54:8–10.
Benchikh A, Savage C, Cronin A, et al. A panel of kallikrein markers can predict outcome of prostate biopsy following clinical work-up: an independent validation study from the European Randomized Study of Prostate Cancer Screening, France. BMC Cancer. 2010;10:635.
Gupta A, Roobol MJ, Savage CJ, et al. A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer Screening in Rotterdam, Netherlands. Br J Cancer. 2010;103(5):708–14.
Cao DL, Ye DW, Zhang HL, Zhu Y, Wang YX, Yao XD. A multiplex model of combining gene-based, protein-based, and metabolite-based with positive and negative markers in urine for the early diagnosis of prostate cancer. Prostate. 2011;71:700–10.
Clark J, Merson S, Jhavar S, et al. Diversity of TMPRSS2-ERG fusion transcripts in the human prostate. Oncogene. 2007;26:2667–73.
Laxman B, Morris DS, Yu J, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645–9.
Petrovics G, Liu A, Shaheduzzaman S, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24:3847–52.
Stephan C, Kahrs AM, Cammann H, et al. A [−2]proPSA-based artificial neural network significantly improves differentiation between prostate cancer and benign prostatic diseases. Prostate. 2009;69:198–207.
Guazzoni G, Nava L, Lazzeri M, et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/mL: results of a prospective study in a clinical setting. Eur Urol. 2011;60:214–22.
Acknowledgments
No sources of funding were used to prepare this article. The authors have no conflicts of interest that are directly relevant to the content of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansen, J., Rink, M., Graefen, M. et al. Assays for Prostate Cancer. Mol Diagn Ther 17, 1–8 (2013). https://doi.org/10.1007/s40291-013-0014-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-013-0014-y